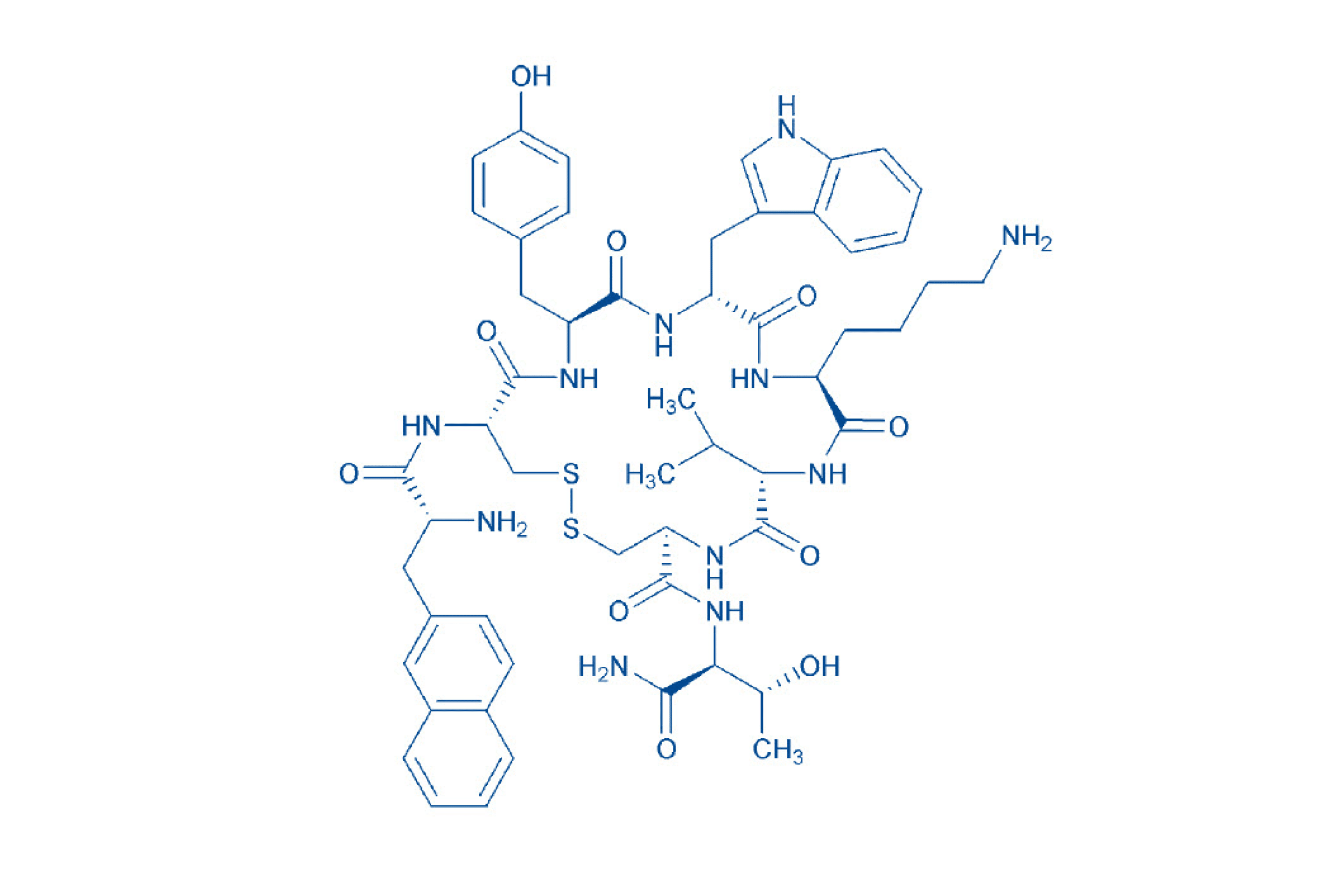
Lanreotide
H-9055-GMP, 4045923
What is lanreotide?
Lanreotide is a cyclopeptide of 8 amino acids that belongs to a class of drugs known as somatostatin analogs. Somatostatin plays a crucial role in regulating various physiological functions in the body.
It is produced by the delta cells of the pancreas, as well as by other cells in the gastrointestinal tract and the hypothalamus region of the brain. Similar to octreotide, lanreotide is used for the treatment of conditions caused by an increased production of growth hormones, most notably acromegaly and neuroendocrine tumors. As a drug, lanreotide is marketed as the acetate salt of lanreotide and branded under the name Somatuline® Depot or Autogel by Ipsen.
What are acromegaly and neuroendocrine tumors?
Acromegaly
Acromegaly is a rare hormonal disorder that occurs when the pituitary gland produces excessive amounts of growth hormone (GH). This excess of GH leads to an overproduction of another hormone called insulin-like growth factor 1 (IGF-1). Both GH and IGF-1 play crucial roles in regulating growth, especially during childhood and adolescence. When the overproduction occurs in adulthood, it usually results a noncancerous tumor of the pituitary gland, known as a pituitary adenoma exerting overproduction of GH and IGF-1. The secretion of elevated IGF-1 level contributes then to the clinical manifestations of acromegaly.
The main characteristics and features of acromegaly include:
- Enlargement of bones and tissues: One of the hallmark signs of acromegaly is the abnormal growth of bones and tissues. This typically leads to the enlargement of the hands and feet, as well as facial features such as the nose, jaw, and forehead. Soft tissues, such as the tongue and internal organs, may also grow larger.
- Coarsening of facial features: Individuals with acromegaly often develop thick facial features due to the growth of bones and tissues. This can result in a protruding jaw, enlarged nose, thickened lips, and widely spaced teeth.
- Joint and soft tissue changes: Enlarged bones and tissues can cause joint pain and limited joint mobility. Soft tissues, such as the skin, can thicken, resulting in a coarse oily appearance.
- Internal organ enlargement: Internal organs may also be affected, leading to complications such as cardiomegaly (enlarged heart), sleep apnea, and gastrointestinal issues.
- Increased sweating and oily skin: People with acromegaly often experience excessive sweating and have oily skin.
- Headaches and vision problems: In some cases, the enlarged pituitary gland can press on nearby structures, leading to headaches, vision problems, and, in severe cases, loss of peripheral vision.
Neuroendocrine tumors
Neuroendocrine tumors (NETs) are a group of rare tumors that develop from cells of the neuroendocrine system. The neuroendocrine system is a network of cells that release hormones into the bloodstream in response to signals from the nervous system. In many patients with NETs, hypersecretion of neuropeptides represents a major clinical problem and leads to carcinoid syndrome (CS). These tumors can occur in various organs throughout the body, including the pancreas, lungs, gastrointestinal tract, and other areas.
The treatment for neuroendocrine tumors depends on several factors, including the location of the tumor, its size, whether it has spread to other parts of the body, and the overall health of the patient. Though curative surgery in neuroendocrine tumors is possible, the CS is mostly associated with liver metastases making the treatment-options non-curative in most cases. Different palliative therapeutic options are possible for the patients. Somatostatin analogs are a common treatment in these forms of NETs. Unfortunately, these therapies do not eradicate the tumor, however they are able to improve health related quality of life by reducing the symptoms of CS in these patients.
How does lanreotide work?
Lanreotide mechanism of action
Lanreotide acetate, as a somatostatin analog, mimic somatostatin and act on somatostatin receptors particularly abundant on different zone of the body: pituitary gland, pancreas, gastrointestinal (GI) tract. When somatostatin analogs bind to these receptors, they exert inhibitory effects on hormone secretion, including the following:
- In the pituitary gland, it inhibits the release of GH. This is particularly important in the treatment of acromegaly, where excessive GH production leads to the overproduction of IGF-1, resulting in abnormal growth of tissues and organs. Lanreotide helps normalize these hormonal imbalances.
- It can reduce the secretion of insulin from the pancreas and glucagon from the alpha cells of the pancreas. This can be beneficial in conditions like insulinomas, where excess insulin production causes hypoglycemia.
- It also inhibits the release of gastrin, a hormone that stimulates gastric acid. This action can be useful in managing conditions associated with excessive gastric acid production.
The action of lanreotide acetate on GH secretion has a direct effect on tumor growth inhibition. In the case of neuroendocrine tumors, the action on somatostatin receptors can inhibit the growth and secretion of certain tumors that express these receptors. This can help slow down the progression of the disease and alleviate symptoms associated with hormone hypersecretion.
Finally, by reducing the levels of hormones such as GH and IGF-1, lanreotide helps alleviate symptoms associated with acromegaly, such as abnormal growth of bones and tissues, soft tissue swelling, and other related complications as well as improve patients quality of life in the case of NET.
Complex formulation for a long-sustained release of lanreotide
The formulations of somatostatin analogs have been improved over time and new longer acting versions were introduced, Sandostatin LAR® Depot for octreotide and Somatuline® Depot or Autogel for lanreotide.
The original formulation of lanreotide was a prolonged release (PR) or sustained release (SR) that utilized a microparticle vehicle to sustain the effect of the drug once administered. The PR formulation was administered every 7-14 days. However, microsphere-based formulations have inherent disadvantages, including the need for reconstitution before injection, a complex manufacturing process requiring the use of organic solvents that are often toxic, potential burst release of the drug, the generation of acidic metabolites during the polymer degradation process, and possible degradation of the peptide. Moreover, microsphere-based formulations often required potentially painful intramuscular injections as well as large injection volumes that can limit the amount of drug administered at a time and hence the length of drug exposure. Thus, an innovative approach for a new formulation of lanreotide with an improved pharmacokinetic profile and free of these disadvantages was developed.
Lanreotide became available in a preparation that delivered more sustained levels of drug through a depot formulation. This formulation is a supersaturated gel of lanreotide acetate and water only. The supersaturated gel is produced by self-assembly of lanreotide. The process starts with noncovalent peptide dimerization, formation, and growth of an open ribbon and ends with ribbon closure in the shape of a nanotube. The structure of the semi-solid gel results from a very dense packing of the nanotubes. The nanotube assembly is completely reversible. At low lanreotide concentration, the nanotubes slowly disassemble, releasing the active drug. The slow and controlled drug release starts immediately upon transfer into a dilute solution like when injected into the body. 1Wolin, E. M. et al. J. Gastrointest. Canc. 47, 366–374 (2016).
Somatuline® Depot or Autogel contains lanreotide acetate in a prefilled syringe that is fitted with an automatic needle guard. The formulation is based on the peptide self-assembly, which provides an innovative solution to sustain a therapeutic dose over a long period of time. Indeed, Somatuline® Depot is the first marketed sustained-release formulation produced via peptide self-assembly, which enables a stable linear release profile over the following 28 days.
| Brand name | Company | Indication | Dosage | Formulation / Route of administration | Year of approval |
|---|---|---|---|---|---|
| Somatuline®Depot/ Autogel | Ipsen | Acromegaly, GEP-NET*, CS | 60 mg/0.2 mL, 90 mg/0.3 mL, and 120 mg/0.5 mL | SC** injection pre-filled syringe (redisigned system) | 2007 |
| Mytolac®/ Myrelez (generic) | Advanz Pharma | Acromegaly, GEP-NET* | 90 mg/0.3 mL, and 120 mg/0.5 mL | SC** injection pre-filled syringe | 2021 |
| Lanreotide generic | Cipla | GEP-NET* | 120 mg/0.5 mL | SC** injection pre-filled syringe | 2021 |
* GEP-NET: gastroenteropancreatic neuroendocrine tumor
** SC: subcutaneous
In 2021, two generic drugs of Somatuline® Depot have been approved in the US and in Europe and commercialized by Cipla and Advanz Pharma. However, unlike the new device developed by Ipsen to facilitate the injection of the supersaturated gel, both lanreotide generics use the previous system of syringes.
How is lanreotide produced?
Benefit from Bachem experience in somatostatin analogs manufacturing
Lanreotide is a cyclic peptide of 8 amino acids containing a disulfide bridge between the two cysteines. With this complex structure, experience in peptide synthesis is key to develop an efficient manufacturing process that will deliver a consistently high-quality product. Manufactured in our site in the US, we have successfully completed a technology transfer to increase the batch size up to 5kg and to meet larger volume demand. With our extensive experience in peptide synthesis, you benefit from an efficient and controlled manufacturing process providing high purity lanreotide API with a tight control on the acetate content and particle size distribution. Both characteristics can have an influence on the solubility of the API and therefore impact the self-assembly mechanism of the drug product during the formulation. The quality of lanreotide is of utmost importance to enable the consistent formation tridimensional structure.
With Bachem experience, our customers unlock excellence in the treatment of acromegaly and NET with Bachem’s lanreotide acetate.

Your partner of choice to simplify complexity
As the leading CDMO in complex peptide manufacturing, we recognize the significance of innovation to improve efficiency, resilience, and sustainability. We provide customer-centric services designed to streamline and improve our customer’s lives. From our rich experience, we understand that time, regulatory assistance, and quality are crucial factors for our customers’ success.
Expertise in somatostatin analogs: Benefit from our specialized knowledge in somatostatin analogs with a proven track record of quality for complex peptide API.
Ready for complex formulation: our customers can rely on our lanreotide API for intricate formulations. Our product displays high purity with meticulous control of acetate content, ensuring the precision your drug product demands.
DMF approved in the US: Trust in the regulatory excellence, supported by a Drug Master File (DMF) approved in the United States to streamline your regulatory submission. Our commitment to quality meets the highest industry standards.
First-class service support: Elevate your development journey with all-around Bachem services. Our dedicated team ensures a seamless experience from inception to delivery, prioritizing your project’s success.
Accelerate your timelines: ensure a swift access to the resources and the flexibility tailored to your need with our material on stock and available impurities.
List of impurities and related products:
| Product number | Name | Sequence |
|---|---|---|
| 4067760 | Lanreotide | H-D-2-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH₂ |

Bachem Regulatory Documentation
DMF
Lanreotide synonyms
BIM-23014; DC-13-116;
(D-2-Nal5,Cys6,11,Tyr7,D-Trp8,Val10)-Somatostatin (5-12) amide
Lanreotide sequence
H-D-2-Nal-Cys-Tyr-D-Trp-Lys-Val-Cys-Thr-NH2 (Disulfide bond)
Molecular Information
Molecular Formula
C54H69N11O10S2
Relative Molecular Mass
1096.34 g/mol
CAS-Number
108736-35-2 (net), 127984-74-1 (acetate)
Long-term Storage
-20 ± 5°C
Contact US
- 1Wolin, E. M. et al. J. Gastrointest. Canc. 47, 366–374 (2016).