MEET US AT TIDES, APRIL 30 – MAY 3, 2017 IN SAN DIEGO, CALIFORNIA
TIDES is the largest meeting to accelerate promising oligonucleotide and peptide molecules from research to commercialization. The 2017 edition will take place on April 30 – May 3 at the Manchester Grand Hyatt San Diego, San Diego, CA.
TIDES brings 850+ global oligonucleotide and peptide leaders across Asia, Europe and North America together to present case studies, best practices and to discuss current strategies and trends to accelerate promising molecules to market. Do not miss the following presentations of the Bachem experts:
– Pre-Conference Workshop 1:
Dr. Tobias Hauck, VP Quality Control, Bachem AG, “A Holistic Quality Control Strategy for Peptide APIs” at 8:30 am – 9:15 am
Sumukh Ray, Method Development Manager, Bachem Americas Inc., “MS Peak Homogeneity: The Use of Mass Spectrometry as a Tool for LC Method Specificity” at 10:30 am – 11:00 am
– Main Conference, Peptide Chemistry Manufacturing and Controls, Day Two:
Dr. Dirk Bächle, Director Quality Control II, Bachem AG, “High Quality Starting Materials: One Important Key to Success” at 8:30 am – 9:00 pm
Dr. Gerhard Haas, VP Quality Assurance and Regulatory Affairs, Bachem AG, “Regulatory Change Control for Peptide Starting Materials: A Case Study” at 9:30 am – 10:00 pm
Bachem‘s pipeline contains more than 150 customer projects in preclinical and clinical phases. Recently, some of our projects in phase III trials received marketing authorization and phase II projects progressed to pivotal phase III clinical trials.
Bachem’s services include pegylated peptides, lipidated peptides, various other peptide conjugates, sterile fill and finish (Clinalfa®), and selective chemical glycosylation. The glycosylation technology is applicable to large scale and has the potential to be applied to a variety of peptides, where we can pioneer the concept of improving current and future drugs. To view our webinar please click here.
We are excited to meet with our customers and discuss how Bachem can help with their peptide API custom manufacturing needs. We invite you to visit us at our Booth #203: please contact us to schedule a meeting in advance.
We look forward to meeting you at TIDES 2017!
CHEMICAL GLYCOSYLATION OF PEPTIDES
Introduction
Glycosylation involves covalent attachment of carbohydrates to macromolecules such as lipids, proteins, and peptides. Glycosylated products can be classified into five major classes namely, N-linked glycans (has carbohydrate part attached to nitrogen of asparagine or arginine side-chains), O-linked glycans (attached via the hydroxyl oxygen of serine, threonine, tyrosine, hydroxylysine, or hydroxyproline side-chains, or to oxygens on lipids such as ceramide), Phospho-glycans (linked through the phosphate group of a phospho-serine), C-linked glycans (where a sugar is added to a carbon on a tryptophan side-chain and Glypiation (formed by the addition of a glycosylphosphatidylinositol, GPI, anchor that links proteins to lipids through glycan linkages). A number of proteins are not stable unless they contain oligosaccharides linked at the amide nitrogen of asparagine. It has been estimated that as much as 50% of all proteins are glycosylated. The influence of glycosylation on the folding and stability of glycoprotein is twofold. Firstly, the highly soluble glycans may have a direct physicochemical stabilization effect. Secondly, N-linked glycans mediate a critical quality control check point in glycoprotein folding in the endoplasmic reticulum. Glycosylation is an important parameter in the optimization of many glycoprotein-based drugs such as monoclonal antibodies [1].
During drug development, chemical modifications are often introduced to stabilize molecules with short half-lives. One such strategy consists of modifying protein and peptide drugs with polyethylene glycol (PEG) to achieve increased plasma life time and reduced immunogenicity [2]. However, PEGs have not been reported to significantly stabilize proteins thermodynamically. Also, PEGs are not biodegradable and reported to have tendency to accumulate in the body [3]. In recent years, glycosylation has been evolved as an alternative strategy to improve bioavailability of peptides. Thus, the recombinant glycosylation technology used to stabilize commercially available medicines (i.e., glycosylated erythropoietin-α (ARANESP®) and glycosylated brain natriuretic peptide (NATRECOR®)) [4] leads very often to a heterogeneous mixture of glycoforms.
Glycosylated peptides
Glycosylation can improve the physicochemical properties of peptides (see figure 1 as an example of glycopeptide that functions as a hormone). These include solubility, stability, half-life and homogeneity. Glycosylation can also have positive effects on pharmacological properties of a peptide-drug candidate. Glycosylation could impart better binding to receptors, modify receptor selectivity, and impart better drug tolerance. Other advantages of peptide glycosylation include: targeting specific organs and enhancing bio-distribution in tissues, improving penetration through biological membranes, increasing metabolic stability and lowering the clearance rate, protecting amino acid’s side chain from oxidation, maintaining and stabilizing the physical properties of peptides, such as precipitation, and aggregation and thermal and kinetic denaturation [5]. Conjugation of sugars with peptides can also facilitate the active transport of modified compounds across cell membranes by targeting glucose transporters on the surface of biological membranes.
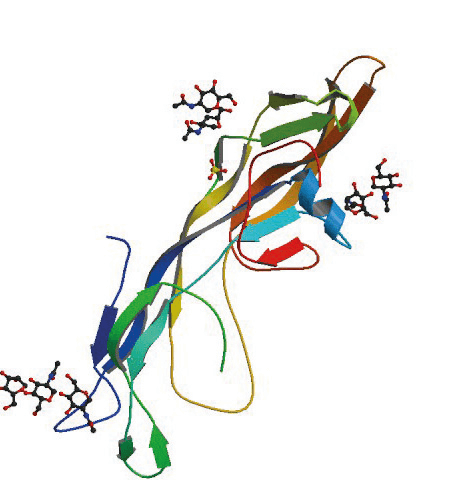
Figure 1: Human follicle-stimulating hormone, a 92 amino acid glycopeptide
PDB: 1FL7
Fox, K.M., Dias, J.A., Van Roey, P.
Three-dimensional structure of human follicle-stimulating hormone
Mol. Endocrinol.15: 378-389 (2001)
Perhaps the most important feature of chemical glycosylation is its selectivity: one can select the position(s) of glycosylation within the peptide, as well as specify the exact glycan that is bound to an individual amino acid. This level of control makes chemical glycosylation more powerful compared to traditional glycosylation technology that utilizes recombinant expression. Biological systems often produce a heterogeneous mixture of glycoforms [6] (Figure 2). Chemical synthesis can be used to obtain homogeneous glycans in large quantities. Enzymes can be used together with chemical methods to further simplify the process of glycan synthesis.
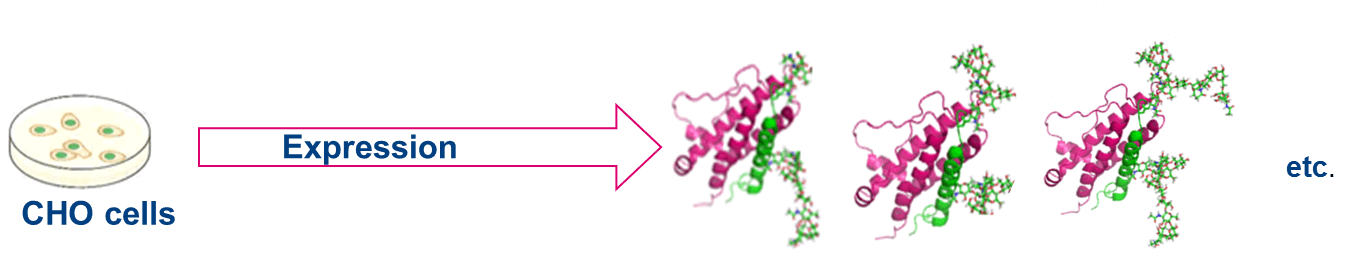
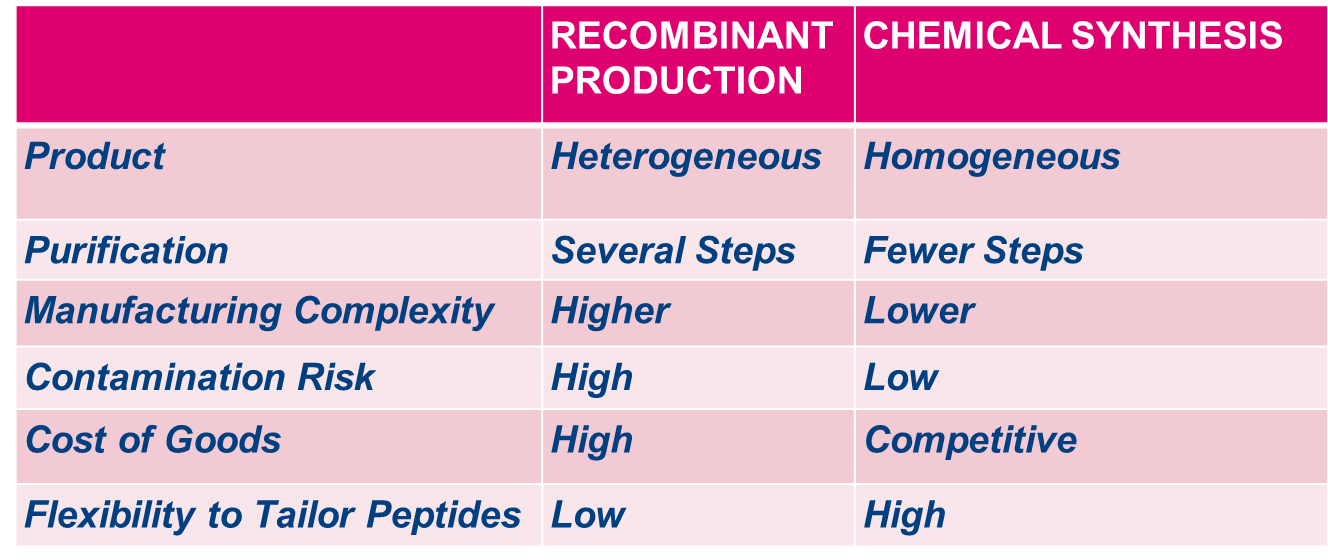
Figure 2: Chemical glycosylation vs recombinant technology: Biological systems often produce a heterogeneous mixture of glycoforms whereas chemical synthesis can be used to obtain homogeneous glycans in large quantities.
Design of glycosylated peptides
N-linked glycosylation is the most common form of glycosylation – where the carbohydrate is linked via amide bond to an asparagine (Asn) side chain of a peptide. During the design, the position of glycosylation on the peptide is identified empirically based on the desired impact of the glycan (half-life extension, tissue targeting, etc.). Even if the peptide has multiple asparagine residues in the sequence, it is possible to selectively glycosylate at a specific position. If the peptide doesn’t have an asparagine residue, it would be possible to glycosylate through the side chains of other native residues such as cysteine and lysine. It is also possible to insert a non-native cysteine residue if necessary, and a library of different glycosylated peptides can be easily generated. Just like controlling the position of glycosylation, it is also important to control the number of glycans, or number of positions that are glycosylated to achieve desired manipulation of pharmacological properties. Once the position(s) of glycosylation and type of glycan are finalized, chemical glycosylation reaction is performed to afford glycosylated peptide (Figure 3).
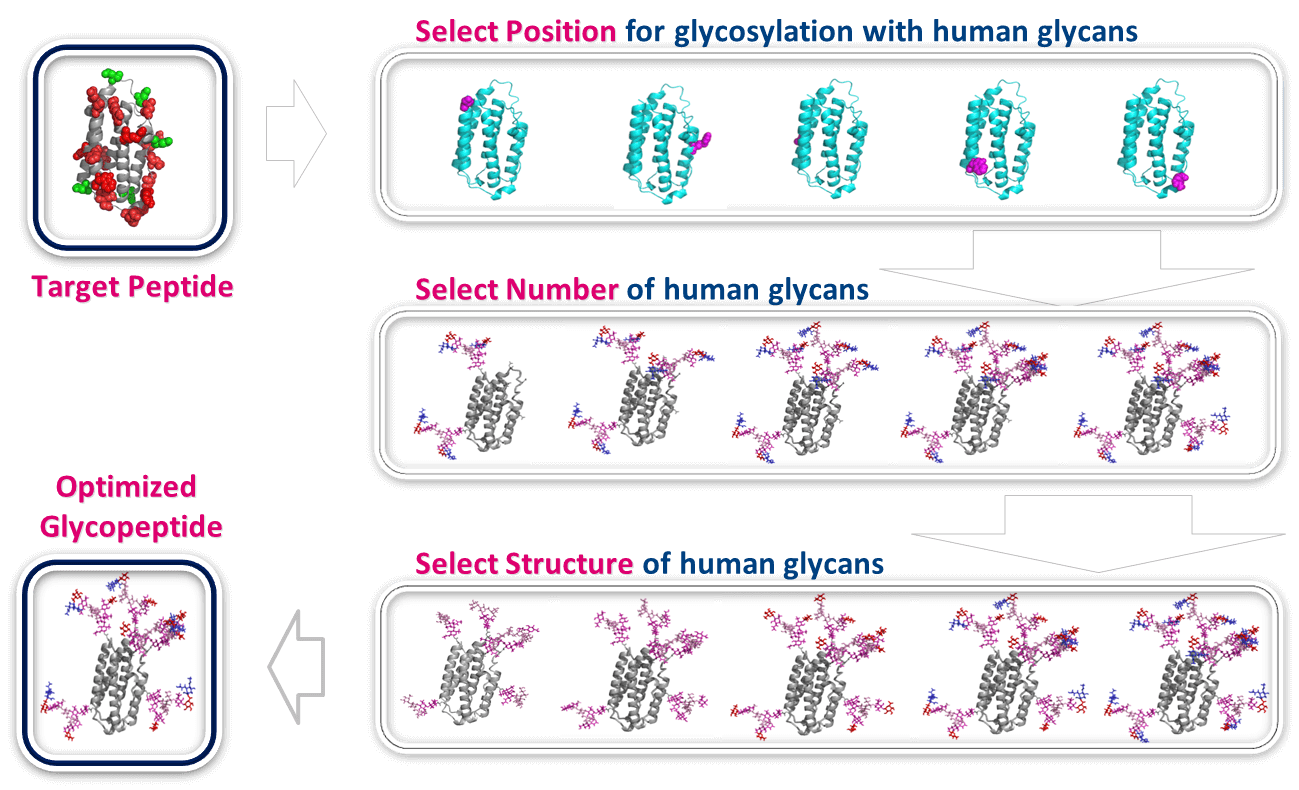
Figure 3: Design of glycosylated peptides
Synthesis of glycosylated peptides
Direct and convergent syntheses are two common chemical strategies for the synthesis of N- or O-linked glycopeptides. In the direct method, the pre-synthesized glycosylated amino acid is coupled to the elongating peptide using solid phase peptide synthesis (SPPS) in a stepwise fashion. Two methods, fluorenylmethyloxycarbonyl (Fmoc) and tert-butyloxycarbonyl (Boc) chemistry are used in SPPS, the Fmoc- method being the method of choice for glycosylated peptides. Typically, the hydroxyl groups of the glycan component are protected as acetyl or benzoyl esters, which are easily removed with base after the completion of peptide assembly. Convergent (fragment-condensation) methods including on-resin linked glycopeptide and Lansbury aspartylation are used for making peptides with long sequences (>50 residues). Chen and Tolbert reported an on-resin convergent synthesis in which 2-phenylisopropyl protecting group is used as an orthogonal handle to create glycosylation sites on-resin for the coupling of a large high mannose oligosaccharide to peptides to suppress the aspartimide formation [7]. Introducing allyl esters and 4-[N-[1-(4,4-dimethyl-2,6-dioxocyclohexylidene)-3-methylbutyl]-amino]benzyl (Dmab) as protecting groups on aspartic acid residues is also an efficient method for selective deprotection and improving the yield of N-linked glycopeptide 8]. Wang et al. reported a modified Lansbury aspartylation for the synthesis of complex glycopeptides [9]. In this method, short glycopeptide fragments were synthesized using convergent aspartylation followed by ligation with a long peptide domain, in which a pseudoproline motif was incorporated into Ser or Thr residues to inhibit the production of aspartimide by-products. The enzymatic transfer of individual monosaccharides to preformed glycopeptides is an excellent alternative to the synthesis of large glycosyl–amino acids. After glycopeptide synthesis using a glycosyl–amino acid cassette, the glycan is deprotected and then elaborated with glycosyltransferases. The synthesis of a PSGL-1 fragment is an elegant example of using both chemical and enzymatic methodologies to generate a complex glycopeptide [10]. In addition to O- and N-linked glycosylation approaches, several chemical methods have been established for the attachment of carbohydrate units to different amino acid residues at the N-terminus of the peptide’s sequence [11]. Conjugation of galactose to the N-terminus of α-melanocyte-stimulating hormone octapeptide analogue (NAPamide) is one of the examples. The carbohydrate is carboxymethylated at the anomeric carbon und thus can be attached to the N-terminus of NAPamide during SPPS [12]. N-terminal modification of peptides is also achievable by conjugation of carbohydrate units to peptide through a succinamic linker, in which the azide derivative of the sugar moiety is replaced by succinamic acid at the anomeric carbon and coupled to the N-terminus of the peptide through a peptide bond [13].
Libraries of well characterized glycans are currently commercially available (GlyTech Inc.) to be used for glycosylation of peptides. Figure 4 lists a number of human type asparagine linked glycans. Use of such highly purified glycans is desirable for manufacturing homogeneous glycopeptides and glycoproteins with optimized glycan structures at desired glycosylation positions.
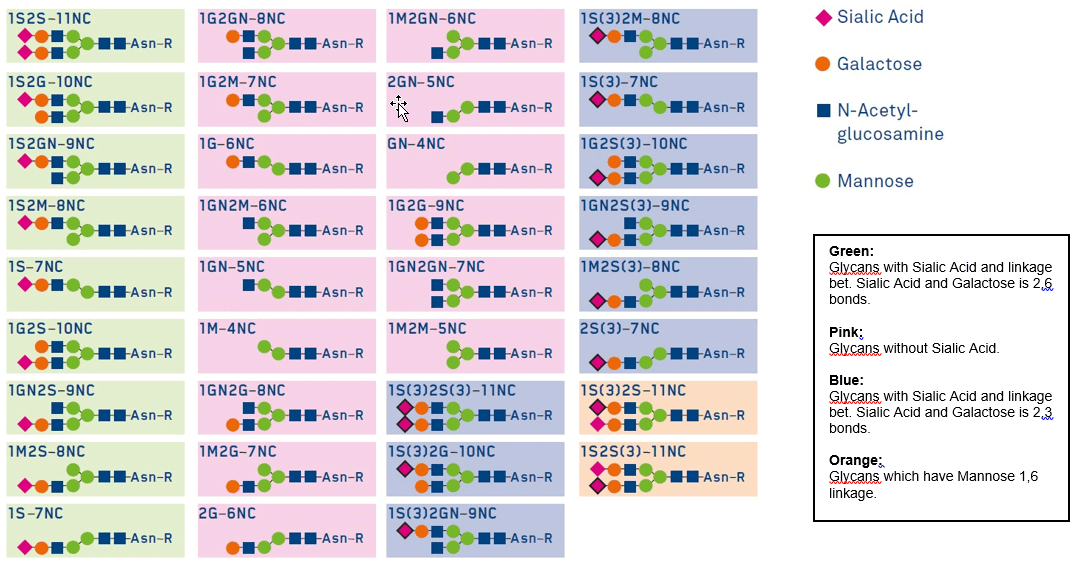
Figure 4: Library of highly purified and well characterized human type asparagine linked glycans
The hydroxyl groups of these glycans are free (no protection required) and also are compatible with Fmoc or Boc strategies. These can be incorporated in peptides during solid phase peptide synthesis (Figure 5).
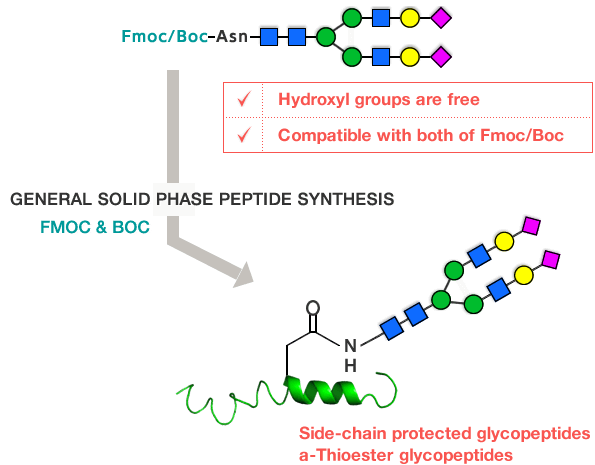
Figure 5: Incorporation of glycans during peptide synthesis
Synthesis of interferon-β
Kajihara’s group in Japan has successfully synthesized homogeneous Human Glycosyl-interferon-β via native chemical ligation [14]. The 166 residue polypeptide chain of interferon-β, glycosylated at Asn80, was prepared by covalent condensation of two synthetic peptide segments and a glycosylated synthetic peptide bearing a complex-type glycan of biological origin. The peptides were covalently condensed by native chemical ligation. Selective desulfurization followed by deprotection of the two Cys(Acm) residues gave the target full-length polypeptide chain of interferon-β bearing either a complex-type sialyl biantennary oligosaccharide or its asialo form. Subsequent folding with concomitant formation of the native disulfide bond afforded correctly folded homogeneous glycosyl-interferon-β. The chemically synthesized sialyl interferon-β exhibited potent antitumor activity in vivo. This work shows that chemical synthesis of homogeneous human glycoproteins exhibiting bioactivity in vivo is possible.
Conclusions
Glycosylation has been shown to markedly improve drug properties such as half-life, binding affinity and selectivity. It can be a powerful way to optimize pharmacological properties of a lead drug candidate. Selective glycosylation, via chemical synthesis, leads to a homogeneous product with potential for better defined bioactivity compared to heterogeneous glycoforms possible in recombinant synthesis. New glycosylation technology permits the synthesis of selectively glycosylated peptides and proteins via incorporation of glycan-derivatized amino acid building blocks during the course of SPPS and LPPS. A proprietary library of highly purified and well characterized glycan-derivatized amino acids is now commercially available to support the development of peptides and proteins with enhanced physiochemical and pharmacological properties.
References
(1) Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA, Emerging principles for the therapeutic exploitation of glycosylation. Science 2014, 343: 1235681.
(2) Veronese FM, Pasut G, PEGylation, successful approach to drug delivery. Drug Discov Today 2005, 10: 1451-1458.
(3) Knop K, Hoogenboom R, Fischer D, Schubert US, Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl 2010, 49: 6288-6308.
(4) Leader B, Baca QJ, Golan DE, Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov 2008, 7: 21-39.
(5) Moradi SV, Hussein WM, Varamini P, Simerska P, Toth I, Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem Sci 2016, 7: 2492-2500.
(6) Park SS, Park J, Ko J, Chen L, Meriage D, Crouse-Zeineddini J, Wong W, Kerwin BA, Biochemical assessment of erythropoietin products from Asia versus US Epoetin alfa manufactured by Amgen. J Pharm Sci 2009, 98: 1688-1699.
(7) Chen R, Tolbert TJ, On-resin convergent synthesis of a glycopeptide from HIV gp120 containing a high mannose type N-linked oligosaccharide. Methods Mol Biol 2011, 751: 343–355.
(8) Conroy T, Jolliffe KA, Payne RJ, Synthesis of N-linked glycopeptides via solid-phase aspartylation. Org Biomol Chem 2010, 8: 3723–3733.
(9) Wang P, Aussedat B, Vohra, Y Danishefsky SJ, An advance in the chemical synthesis of homogeneous N-linked glycopolypeptides by convergent aspartylation. Angew Chem Int Ed Engl 2012. 51: 11571-11575.
(10) Koeller KM, Smith MEB, Huang RF, Wong CH, Chemoenzymatic Synthesis of a PSGL-1 N-Terminal Glycopeptide Containing Tyrosine Sulfate and α-O-Linked Sialyl Lewis X. J Am Chem Soc 2000, 122: 4241–4242.
(11) Moradi SV, Mansfeld FM Toth I, Synthesis and in vitro evaluation of glycosyl derivatives of luteinizing hormone-releasing hormone (LHRH). Bioorg Med Chem 2013, 21: 4259–4265.
(12) Bapst JP, Calame M, Tanner H, Eberle AN, Glycosylated DOTA-alpha-melanocyte-stimulating hormone analogues for melanoma targeting: influence of the site of glycosylation on in vivo biodistribution. Bioconjug Chem 2009, 20:984-993.
(13) Ziora ZM, Wimmer N, New R, Skwarczynski M, Toth I, Synthesis of glycolipopeptidic building blocks for carbohydrate receptor discovery. Carbohydr Res 2011, 346:1439-1444
(14) Sakamoto I, Tezuka K, Fukae K, Ishii K, Taduru K, Maeda M, Ouchi M, Yoshida K, Nambu Y, Igarashi J, Hayashi N, Tsuji T, Kajihara Y, Chemical synthesis of homogeneous human glycosyl-interferon-beta that exhibits potent antitumor activity in vivo. J Am Chem Soc 2012, 134:5428-5431.
CHEMICAL GLYCOSYLATION IN CLINICAL DEVELOPMENT
Many proteins and peptides in living cells are glycosylated and play key roles in structure, protection, lubrication, reproduction, adhesion, the immune system and other biological functions. The synthesis of glycopeptides has allowed researchers to study the function of glycans and identify products that have useful therapeutic applications. For example, natural and semi-synthetic glycopeptide antibiotics have been developed as drugs for the treatment of life-threatening infections caused by multi-drug resistant Gram-positive pathogens like Staphylococcus aureus, Enterococcus spp. and Clostridium difficile (1). In addition, glycosylation of peptide drugs has gained interest as a strategy to optimize drug candidates and can result in improved drug properties such as increased half-life, enhanced solubility and improved bioactivity (2).
There are several glycopeptide antibiotics and other glycopeptides in various phases of clinical development as shown in Table 1.
Table 1: Glycopeptides in Phase I to Phase III Clinical Development
| Product Name | Active Ingredient | Condition Treated | Highest Phase | Companies |
|---|---|---|---|---|
| NeoPulse Program | bleomycin | Head and Neck Cancer(III) Skin Cancer(III) | Phase III | OncoSec Medical Inc |
| TD1792 | cefilavancin | Skin Bacterial Infections(III) Pneumonia(I) | Phase III | Innoviva, Theravance Biopharma U.S Inc, R-Pharm |
| AeroVanc | vancomycin hydrochloride | Staphylococcal Infections(I) Pneumonia(PC) | Phase II | Savara Pharmaceuticals, Mast Therapeutics Inc |
| Blenoxane PCI | amphinex, bleomycin | Solid Tumors(I) | Phase II | PCI Biotech AS |
| ONT10 | glycosylated mucin 1, cell surface associated fragment (Liposomal) | Metastatic Breast Cancer(I) Metastatic Ovarian Cancer(I) Solid Tumors(I) | Phase I | Cascadian Therapeutics Inc, Immunovaccine Inc, Celldex Therapeutics |
| PKX001 | -- | Insulin-Dependent Diabetes Mellitus(I) Dermatology(PC) Inflammatory Disorders(PC) | Phase I | ProtoKinetix Inc, University of Alberta |
| TD1607 | -- | Bacterial Infections(I) | Phase I | Innoviva, Theravance Biopharma U.S Inc |
Phase III Candidates
OncoSec Medical is developing the NeoPulse Program to treat head and neck cancer and skin cancer. NeoPulse involves the combined use of electroporation with bleomycin. Electroporation involves the use of electrical impulses to increase the permeability of the cell membrane and bleomycin is a cytotoxic glycopeptide antibiotic which inhibits DNA synthesis to treat tumors. In 2012, OncoSec Medical announced positive interim results from a Phase IV trial studying the use of NeoPulse in skin cancer patients. In addition, the company announced positive interim results from a Phase IV study investigating the use of NeoPulse in primary and locally recurrent squamous cell carcinoma of the head neck (3).
Theravance BioPharma, is developing TD1792 (cefilavancin), a glycopeptide-cephalosporin (beta-lactam) heterodimer antibiotic, in partnership with R-Pharm. The product is being developed for the treatment skin bacterial infections and pneumonia. R-Pharm initiated a Phase III clinical study of TD1792 in complicated skin and soft tissues infections caused by gram-positive bacteria (3).
Phase II Candidates
Savara Pharmaceuticals is developing AeroVanc (vancomycin hydrochloride) for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections and ventilator associated pneumonia (VAP). AeroVanc is an inhaled dry powder form of vancomycin, a bacterial cell wall synthesis inhibitor (3). The company is planning to start enrollment of patients in a Phase III clinical trial of AeroVanc in cystic fibrosis patients with chronic MRSA infections in Q1 of 2017 (4).
PCI Biotech is developing Blenoxane PCI as a treatment for solid tumors. Blenoxane contains amphinex, a photosensitizer, and the glycopeptide cytotoxic antibiotic bleomycin as active ingredients (3). In 2016, the company reported results from the first-in-man Phase I study with amphinex given at escalating doses in combination with bleomycin. The treatment was found safe and tolerable and significant anti-tumor effects were seen at all dose levels (5).
Phase I Candidates
ONT10 is a synthetic MUC1-based liposomal glycolipopeptide cancer vaccine that was being developed by Cascadian Therapeutics for the treatment of solid tumors, advanced breast cancer and advanced ovarian cancer. The product had reached Phase I clinical trials (3). In November 2015, Oncothyreon, now known as Cascadian Therapeutics, announced that the company does not intend to conduct additional trials with ONT10 (6).
PKX001 is an anti-aging glycopeptide being developed by ProtoKinetix for the treatment of inflammation, non-insulin dependent diabetes and skin disorders. In March 2017, ProtoKinetix announced that the company started a Phase I clinical trial of PKX-001 treated islet cells used in conjunction with the Edmonton Protocol for the treatment of Type 1 diabetes (3).
Theravance Biopharma is developing another glycopeptide known as TD1607. This compound is a glycopeptide-cephalosporin heterodimer antibiotic for the treatment of Gram-positive infections due to resistant strains of Staphylococcus aureus. In 2014, Theravance Biopharma completed a Phase I study to evaluate the safety, tolerability and pharmacokinetics of TD1607 (3).
Conclusion
Glycopeptides have proved to be interesting drug candidates and are often the focus of biological studies. Bachem has partnered with GlyTech to provide chemical glycosylation of peptides using pre-attached sugars from a proprietary library of over 50 specific glycans. In addition, Bachem offers a variety of amino acid derivatives for chemists to use in the chemical synthesis of glycopeptides such as the building blocks Fmoc-Asn(GlcNAc(Ac)₃-β-D)-OH (B-2480), Fmoc-Ser(GalNAc(Ac)₃-β-D)-OH (B-4235), Fmoc-Ser(GlcNAc(Ac)₃-β-D)-OH (B-3865), Fmoc-Thr(GalNAc(Ac)₃-α-D)-OH (B-2570), Fmoc-Thr(GlcNAc(Ac)₃-β-D)-OH (B-4245), Fmoc-Ser(tBu)-Pro-OH (B-4430), Fmoc-Asp(ODmab)-OH (B-3240) and Fmoc-L-thiazolidine-4-carboxylic acid (B-4015).
References
(1) E. Binda et al., Old and new glycopeptide antibiotics: action and resistance, Antibiotics (Basel). 3, 572-594 (2014)
(2) Chemical glycosylation of peptides, Bachem (2015)
(3) Medtrack (2017)
(4) Pipeline, Savara Pharmaceuticals (2017)
(5) Publication of the fimaporfin (Amphinex) first-in-man Phase I study in Lancet Oncology, PCI Biotech (2016)
(6) Oncothyreon Form-10K Annual Report 2015, Cascadian Therapeutics (2015)
MEET BACHEM: NIKOLAJ DYBOWSKI
What is your official job title at Bachem?
Project Manager
How long have you been with Bachem? Where did you work before Bachem?
I joined Bachem two years ago, on April 1st 2015. Before I worked as a Bioinformatician/Statistician for Evotec in Munich.
Briefly, what do you do at Bachem?
I lead interdisciplinary teams engaged in NCE development projects. Together with our customers, we typically develop their peptide products from the preclinical stage through the clinical phases and hopefully to the market.
What is your academic background/degrees or training?
I have a Diploma and PhD in Bioinformatics.
What makes a perfect day for you?
Sun, ≥ 30°C, shorts and no shoes, swimming and a good beer.
What is your business motto?
Be genuinely interested.
What do you like most about your job?
The most rewarding part of being a project manager is that you get to work with many people from different departments at Bachem and customers from all over the world. It is a great experience to see a product mature over the course of a project and seeing how everyone pitches in to achieve this.
Have you had any particular expectation when you came to Bachem and have these been fulfilled?
Yes, I had hoped for a working environment that encourages active participation and taking on responsibility. I am very happy to say, that all my expectations were fulfilled!
What is your preferred peptide?
I like my peptides linear and soluble.
Thank you very much Niko.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Peptide targeting senescent cells restores stamina, fur, and kidney function in old mice-Science Daily
Spider venom peptide could prevent stroke-induced brain damage-Medical News Today
Models help UD researchers calculate best dosage for osteoporosis treatment-University of Delaware
Nanoparticle paves the way for new triple negative breast cancer drug-Queen’s University Belfast
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
X. Li et al.
Structural Analysis of the Glycosylated Intact HIV-1 gp120-b12 Antibody Complex Using Hydroxyl Radical Protein Footprinting.
Biochemistry 56, 957-970 (2017)
A.G. Semenov and A.G. Katrukha
Different Susceptibility of B-Type Natriuretic Peptide (BNP) and BNP Precursor (proBNP) to Cleavage by Neprilysin: The N-Terminal Part Does Matter.
Clin. Chem. 62, 617-622 (2016)
L.B. Pathangey et al.
Aberrant Glycosylation of Anchor-Optimized MUC1 Peptides Can Enhance Antigen Binding Affinity and Reverse Tolerance to Cytotoxic T Lymphocytes.
L.H. Kristensen et al.
Releasing the brakes in coagulation Factor IXa by co-operative maturation of the substrate-binding site.
Biochem. J. 473, 2395-2411 (2016)
Y. Ge et al.
Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis.
