MEET US AT THE DDF SUMMIT 2017 IN BOSTON
We invite you to meet us at the 7th American Drug Delivery & Formulation Summit 2017.
The meeting will take place at the Westin Copley Place Boston Hotel in Boston, MA from August 28 to 29, 2017.
The DDF Summit highlights the most innovative solutions and exciting studies on the greatest challenges in pharmaceutical development. Covering both small molecules and biologics, participants will find new technologies, concepts and case studies in drug delivery and formulation.
Bachem specializes in the development of innovative, efficient manufacturing processes and the reliable production of peptide-based active pharmaceutical ingredients. Clinalfa® is Bachem’s brand for its clinical trial materials and related services. Clinalfa® basic products are sterile freeze-dried peptides readily available from stock. Clinalfa® plus products are available as sterile freeze-dried products or as ready-to-use liquid formulations. Clinalfa® plus products are manufactured and released on a customized basis and can be used in preclinical or clinical studies (clinical trials). Bachem offers related services to Clinalfa® basic and Clinalfa® plus products, i.e. formulation development, compatibility studies, stress test and post-release stability studies, CMC documentation for IMPD and IND, drug master file and other services.
We are excited to meet with our partners, learn their needs for peptides and discuss how Bachem can help to advance their drug delivery and formulation projects. We invite you to visit us at our Booth #8: please contact us to schedule a meeting in advance.
We look forward to meeting you at the DDF Summit 2017 in Boston!
APPLICATIONS WITH TRITIUM-LABELED PEPTIDES
Tritium-labeled compounds are valuable tools in drug discovery and development. For studies with peptides, tritium labeling is often well suited because it does not alter the structure of the peptide thereby preserving the biological properties of the unlabelled parent peptide. Tritium-labeled compounds have several applications such as for gathering early metabolism data and studying the covalent binding of reactive metabolites. In addition, these labeled compounds are utilized in transporter efflux and uptake studies, receptor binding studies, autoradiography and receptor occupancy studies (1). A couple examples of tritium-labeled peptides used in clinical studies are summarized as follows.
[3H]−Semaglutide
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog being developed by Novo Nordisk as a once-weekly treatment for type 2 diabetes (2). The product is currently pending approval. Novo Nordisk completed an open-label Phase I trial to investigate the absorption, metabolism and excretion (AME) of semaglutide in healthy human subjects. The primary outcome measures were designated as the concentration of the major metabolites of [3H]−radiolabeled semaglutide in plasma, urine and feces after receiving a single dose of 0.5 mg [3H]−radiolabeled semaglutide (3). Semaglutide was radiolabeled with tritium in the octadecanedioic acid moiety in the side chain of lysine 26 to allow for tracking of the fatty acid side chain. Results showed that intact semaglutide was the primary component circulating in plasma for the human subjects and nonclinical species. Also, the results suggested that semaglutide is slowly metabolized by proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid side chain and that degradation products are excreted in urine and feces (4).
[3H]−Liraglutide and [3H]−GLP- 1 (7-37)
Liraglutide (Victoza®) is a glucagon-like peptide-1 analog that is the active ingredient in Novo Nordisk’s once-daily treatment approved for type 2 diabetes (2). The company conducted a Phase I trial in healthy subjects to characterize the metabolic profile of liraglutide in plasma, urine and feces after a single dose of 0.75 mg [3H]−radiolabeled liraglutide (5). Liraglutide was specifically labeled with 3H in the palmitic moiety of the side chain. [3H]-radiolabeled native GLP-1 (7–37) was also prepared in order to compare the metabolic profiles and degradation products. GLP-1 (7–37) was labeled in the Tyr19 position. Results from the study showed that liraglutide is metabolized in vitro by dipeptidyl peptidase-IV (DPP-IV) and neutral endopeptidase (NEP) similar to native GLP-1 (7-37) but at a slower rate. In addition, intact liraglutide was not excreted in urine and feces and low levels of metabolites in plasma suggested that liraglutide is completely degraded within the body (6).
Conclusion
Tritium-labeled peptides can be the ideal choice for use in several types of studies. For customers seeking custom tritium-labeled peptides, Bachem has partnered with RC Tritec to combine our areas of expertise in the synthesis of peptides and radiolabeling. For more details, please visit www.rctritec.com or sales@rctritec.com.
References
(1) C.S. Elmore, R.A. Bragg, Isotope chemistry; a useful tool in the drug discovery arsenal, Bioorg. Med. Chem. Lett. 25(2), 167-171 (2015)
(2) Medtrack, (2017)
(3) Trial Investigating the Absorption, Metabolism and Excretion After a Single Subcutaneous Dose of [3H]-Semaglutide in Healthy Male Subjects, ClinicalTrials.gov (2017)
(4) L. Jensen et al., Absorption, metabolism and excretion of the GLP-1 analogue semaglutide in humans and nonclinical species, Eur. J. Pharm. Sci. 104, 31-41 (2017)
(5) Metabolism and Excretion of Liraglutide in Healthy Male Volunteers, ClinicalTrials.gov (2017)
(6) M. Malm-Erjefält et al., Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase, Drug. Metab. Dispos. 38(11), 1944-1953 (2010)
CUSTOM PEPTIDE SYNTHESIS BY BACHEM AND TRITIUM-LABELING IN PARTNERSHIP WITH RC TRITEC AG

RC TRITEC AG is a reliable partner for the preparation of custom tritium-labeled compounds. Bachem, a company specialized in the process development and manufacturing of peptides, and RC TRITEC have partnered to provide custom tritium-labeled peptides. Like Bachem, RC TRITEC is based in Switzerland.
Fig 1: Clients benefit from the support of RC TRITEC’s experienced radiochemists.

RC TRITEC has a profound, long-term experience in custom tritium-labeling of small molecules, peptides and polymers. RC TRITEC has domestic and international collaborations with large and medium-sized pharma companies, CROs and retailers all over the world, thus demonstrating its excellent reputation as a labeling service. RC TRITEC has established comprehensive experience in shipping radioactive goods all over the world and has developed a broad portfolio of labeling strategies. Among them, typically three methodologies are used for labeling of peptides.
Fig 2: RC TRITEC’s expertise in custom tritium-labeling ensures a superior service.

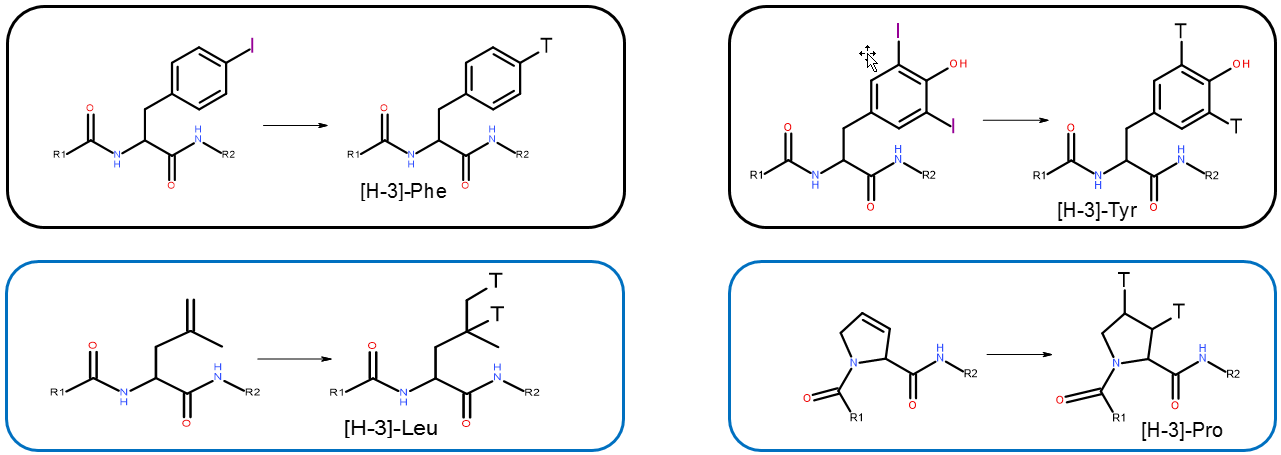
Depending on the peptide sequence, iodine/tritium exchange or hydrogenation of suitable precursor peptides can be used for tritium-labeling of peptides providing high specific activities (refer to the schemes below). In preparation for labeling, Bachem will manufacture each precursor peptide according to RC TRITEC’s proposal and customer requirements. If the sequence contains Phe or Tyr, the corresponding iodo-Phe or di-iodo-Tyr precursor peptide undergoes an I/T exchange and a product with a specific activity of 20-40 Ci/mmol will be obtained. Leu or Pro containing sequences can be labeled by hydrogenation of the unsaturated precursor. The specific activity of these labeled products varies between 20 and 100 Ci/mmol. In case the peptide sequence does not contain any of the amino acids mentioned above, an unselective H/T exchange shall be applied. However, an estimation of the specific activity using this method cannot be made as it is difficult to predict the ratio of incorporation of tritium into the peptide. For more information please contact www.rctritec.com or sales@rctritec.com .
MEET BACHEM: JULIE RISSE
What is your official job title at Bachem?
Business Development Manager
How long have you been with Bachem? Where did you work before Bachem?
I have been with Bachem since September 2016. Before joining Bachem, I was part of the Analytics team at the Silicon Valley Bank in San Francisco. I was working on advisory projects for corporate venture capitals and performing valuations for biotech start-ups.
Briefly, what do you do at Bachem?
I am responsible for the commercial part of NCE development projects. On a daily basis, I take care of customers’ requests and have a lot of interactions with the other departments at Bachem involved in the projects. Part of my role also involves the acquisition of new customers and travelling to scientific fairs and conferences to represent the company.
What is your academic background/degrees or training?
I studied chemistry at EPFL in Lausanne and did my PhD there in organometallic chemistry. I also have a MBA degree from Hult International Business School in San Francisco.
What do you like to do outside of work (interests, hobbies)?
Hiking, running, travelling.
What do you like most about your job?
I like to be in close contact with the customers, it is a privileged position. The friendly working atmosphere at Bachem is also very valuable for me.
Thank you very much Julie.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Peptide half-life dramatically increased using novel approach-GEN
New gonorrhea treatment targets enzyme needed for respiration-Oregon State University
Peptide vaccine shows clinical benefit in glioblastoma-Cancer Network
Designer molecule silences mitochondrial genes-Chemical & Engineering News
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
S. Dvoracsko et al.
Investigation of receptor binding and functional characteristics of hemopressin(1-7).
Neuropeptides 58, 15-22 (2016)
N.B. Borotto et al.
Targeted Annotation of S-Sulfonylated Peptides by Selective Infrared Multiphoton Dissociation Mass Spectrometry.
A. Glöde et al.
Divergent effects of a designer natriuretic peptide CD-NP in the regulation of adipose tissue and metabolism.
R.W. Neier et al.
A ligand-based NMR screening approach for identification and characterization of inhibitors and promoters of amyloid peptide aggregation.
M. Piras et al.
High-Affinity “Click” RGD Peptidomimetics as Radiolabeled Probes for Imaging αv β3 Integrin.
ChemMedChem 12, 1142-1151 (2017)
H. Sui et al.
The SNK and SPAR signaling pathway changes in hippocampal neurons treated with amyloid-beta peptide in vitro.
