MEET US AT CPHI KOREA

Bachem is participating in the CPhI Korea, the leading pharmaceutical event that brings together leaders and key decision makers in South Korea.
The 2017 edition will take place on August 22 – 24, 2017 in Seoul, Korea together with co-located show Hi (Health Ingredients) Korea, which promises a wide range of sourcing opportunities in health, pharma and nutraceuticals.
We are excited to attend for the first time this CPhI event in the pharmaceutical powerhouse of the future! We look forward to meet with our partners and discuss how Bachem can help with their generic API manufacturing needs. With our capacity to produce generic peptide APIs in quantities of hundreds of kilograms and small molecules in tens of tons per year, our Swiss and US GMP manufacturing facilities regularly inspected by the FDA and local authorities, and our record of over 80 DMF filings in the pipeline, we will certainly be able support the success of your projects.
We invite you to visit us at the COEX Convention and Exhibition Centre, Hall D, Booth #E23: please contact us to schedule a meeting in advance.
We look forward to meeting you at CPhI Korea 2017!
CELL CULTURE
Cell culture means growing prokaryotic or eukaryotic cells in vitro under controlled conditions, in an artificial environment. Cells either isolated from tissue (“primary cells”) or obtained from commercial sources are cultivated in vessels containing media providing them with the required nutrients. Cell growth and proliferation are stimulated by growth factors and hormones. Parameters as pH and osmotic pressure of the medium, temperature, composition of the atmosphere (content of oxygen and carbon dioxide), and humidity have to be adjusted and maintained in closed systems. Populations of cells obtained from a cell line are “immortal”, as their proliferation, which started from a single cell and did not stop due to a mutation, is incessant. Such cells (for examples see Table 1) are a popular, low-cost alternative to primary cells, as supply of material from repositories such as ATCC or DSMZ is secure, and homogeneous cell populations ensure reproducible results. Their genome is quite identical (though mutation rate is high; various mutants have been characterized, and cultivated for use in research). Unfortunately, numerous cell lines are misidentified or have been contaminated with other cell lines and mycoplasma.
Table 1: Examples of cell lines used in research or for recombinant protein synthesis.
| Cell Line | Meaning | Species | Source | Morphology |
|---|---|---|---|---|
| A431 | Homo sapiens | epidermoid carcinoma | epithelium | |
| A549 | Homo sapiens | lung adenocarcinoma | epithelium | |
| BHK | (Syrian) baby hamster kidney | Mesocricetus auratus | kidney | fibroblasts |
| Caco-2 | Caucasian colon adenocarcinoma | Homo sapiens | colorectal adenocarcinoma | epithelium |
| CHO | Chinese hamster ovary | Cricetulus griseus | ovary | epithelium |
| COS | CV-1 in origin, carrying SV40 | Chlorocebus aethiops | kidney | fibroblasts |
| EPC | epitheliumioma papulosum cyprini | Pimephales promelas | skin | epithelium |
| HaCat | human adult low calcium high temperature keratinocytes | Homo sapiens | skin | keratinocytes |
| HEK | human embryonic kidney | Homo sapiens | kidney | epithelium |
| HeLa | named after donor Henrietta Lacks | Homo sapiens | cervix carcinoma | epithelium |
| HUVEC | human umbilical vein endothelial cells | Homo sapiens | umbilical vein | endothelium |
| HT1080 | Homo sapiens | fibrosarcoma | conjunctive tissue | |
| Jurkat | named after donor | Homo sapiens | T lymphocyte cells | blood |
| MCF7 | Michigan Cancer Foundation | Homo sapiens | breast cancer | epithelium |
| Sf9, Sf21 | Spodoptera frugiperda | ovar cells | ||
| SH-SY5Y | Homo sapiens | neuroblastoma | bone marrow | |
| Vero | verda reno | Chlorocebus sp. | kidney | epithelium |
Cultures grown from cells isolated from tissue or organs are unique, they contain the genome of their donor. Primary cells are better models for biological systems than cells obtained from cell lines, and they are less prone to mutations. Their cultivation is much more demanding than growing “line” cells, which readily proliferate in commercially available optimized media. The lifespan of primary cells is limited (which not necessarily is a disadvantage), as cells constituting living organisms “age”. Eventually, they cease to divide and undergo apoptosis or autophagy.
For culturing stem cells, i.e. non-differentiated somatic cells, additives suppressing differentiation are required together with stimulators of proliferation.
Cultured cells have found numerous applications, e.g. for studying biological processes in basic medical and life science research and in toxicology, for testing chemicals. Cell cultivation is an essential component of tissue culture and tissue engineering. Intact plants can be grown from cell cultures.
Recombinant protein synthesis requires cultivation of bacteria or eukaryotic cells such as fungi, often on production scale. Cells stemming from mammals or insects allow generation of proteins modified by disulfide bridge formation or glycosylation. For example, the glycoprotein hormone erythropoietin used as drug is produced in cultures of mammalian cells. CHO is an often-used mammalian cell line for recombinant synthesis, genetic modifications allowed the production of glycoproteins. Popular insect cell lines are Sf9 and Sf21.
Cells are grown on an industrial scale (“fermentation”) for producing drugs, monoclonal antibodies, vaccines, or proteins for various uses.
Nutrients and Auxiliaries for Cell Cultivation
The optimal environment for cell growth depends on the type of cells and their application. Tissue cells adhere to surfaces, whereas blood cells are cultivated in suspension. For growth and proliferation, which means synthesizing a plethora of biomolecules such as the constituents of the cell wall, the proteins of the cytoskeleton, or the nucleotides required for DNA replication, cells need a cocktail of nutrients. Sources for carbon, nitrogen, phosphorus and sulfur could be organic molecules such as amino acids and glycosides, or inorganic salts. Type and composition of the added salts also determine the pH of the culture medium. Addition of vitamins is essential for proper growth. Though cells are cultivated under sterile conditions, antibiotics as streptomycin are routinely supplied for eliminating residual microbial contaminants. Cultivation media can be agar-based gels providing the cells with sufficient water. Petri dishes made from glass or plastic are used in standard “two-dimensional” cell culture, whereas hydrogels allow three-dimensional cell growth, which comes closer to biological systems. When growing tumor cells three-dimensionally, a better tumor model is obtained. Hydrogels may mimic the extracellular matrix. They can be formed from hydrophilic natural or synthetic polymers including peptides. Surface modification of synthetic polymers such as crosslinked PEG with adhesive peptides (e.g. RGD peptides) stimulates cell growth additionally (a positive effect can also be observed using dishes coated with fibronectin or laminin). The properties of hydrogels vary over a broad range; the synthetic gels tend to show an improved reproducibility in behavior.
Cells require amino acids as nitrogen source and for expression of proteins, especially the compounds they cannot produce themselves. Cocktails of amino acids or peptides provide them with the required building blocks. Peptone, a popular supplement consisting of short peptides, is obtained by enzymatic digestion of milk or meat proteins. It contains further nutrients such as lipids. Peptone is used as amino acid source in growth media for bacteria. Mixtures of amino acids can also be used as supplements. Besides the 20 proteinogenic amino acids, hydroxyproline and derivatives as N-acetylcysteine are often added. Glutamine, a most important nitrogen source in cell culture, should be added only when starting the culture, as it decomposes. The amino acid cyclizes spontaneously with concomitant formation of toxic ammonia and pyroglutamate. Replacement of glutamine by the dipeptides Ala-Gln or Gly-Gln reduces this risk considerably. N-Acetylation improves the solubility of amino acids such as tyrosine, it is reverted after uptake by cellular enzymes. Well-defined peptides (contrary to peptone) have also been used as supplements.
Cell growth and, especially, proliferation are stimulated by growth factors, which are added in small amounts, e.g. EGF and bFGF for cultivating endothelial cells.
Explore our Broad Offering of Products for Cell Culture
References
C.D. Helgason, C.L. Miller (Eds.), Basic Cell Culture Protocols, Springer 2013
R.R. Mitry, R.D. Hughes (Eds.), Human Cell Culture Protocols, Springer 2012
J. Haycock (Ed.), 3D Cell Culture – Methods and Protocols, Springer 2011
I.A. Cree (Ed.), Cancer Cell Culture – Methods and Protocols, Springer 2011
T. Andersen, P. Auk-Emblem, M. Dornish, 3D Cell Culture in Alginate Hydrogels. Microarrays 2015, 4, 133.
K. Erdmann, N. Grosser, K. Schipporeit, H. Schröder, The ACE inhibitory dipeptide Met-Tyr diminishes free radical formation in human endothelial cells via induction of heme oxygenase-1 and ferritin. J. Nutr. 2006, 136, 2148.
Y. Feng, M. Mrksich, The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry 2004, 43, 15811.
F. Franek, H. Katinger, Specific effects of synthetic oligopeptides on cultured animal cells. Biotechnol. Prog. 2002, 18, 155.
F. Franek, T. Eckschlager, H. Katinger, Enhancement of monoclonal antibody production by lysine-containing peptides. Biotechnol. Prog. 2003, 19, 169.
F. Franek, M. Fussenegger, Survival factor-like activity of small peptides in hybridoma and CHO cells cultures. Biotechnol. Prog. 2005, 21, 96.
F. Franek, Peptides modulate growth and productivity of mammalian cell cultures and suppress apoptosis. BioProcess Int. June 2004, 48.
G. Kaur and J.M. Dufour, Cell lines: Valuable tools or useless artifacts. Spermatogenesis. 2012, 2, 1.
I. Grayson, C. Kessler, Modern applications of amino acids and dipeptides in pharmaceuticals and biopharmaceuticals. Chimica Oggi -Chemistry Today 2015, 33, 46.
C. Hecklau, S. Pering, R. Seibel, A. Schnellbaecher, M. Wehsling, T. Eichhorn, J. Hagen, A. Zimmer, S-Sulfocysteine simplifies fed-batch processes and increases the CHO specific productivity via anti-oxidant activity. J. Biotechnol. 2016, 218, 53.
S. Kang, J. Mullen, L. P. Miranda, R. Deshpande, Utilization of tyrosine- and histidine-containing dipeptides to enhance productivity and culture viability, Biotechnol. Bioeng. 2012, 109, 2286.
J. Kim, R. C. Hayward, Mimicking dynamic in vivo environments with stimuli-responsive materials for cell culture. Trends Biotechnol. 2012, 30, 426.
J.-H. Kim, D.W. Jekarl, M. Kim, E.-J. Oh, Y. Kim, I.Y. Park, J. C. Shin, Effects of ECM protein mimetics on adhesion and proliferation of chorion derived mesenchymal stem cells. Int. J. Med. Sci. 2014, 11, 298.
F. Li, N. Vijayasankaran, A. Shen, R. Kiss, A. Amanullah, Cell culture processes for monoclonal antibody production. MAbs 2010, 2, 466.
D.E. Lynn, Methods for maintaining insect cell cultures. J. Insect Sci. 2002, 2, 9.
Y. Pan, K. E. Webb, Jr., Peptide-bound methionine as methionine sources for protein accretion and cell proliferation in primary cultures of ovine skeletal muscle. J. Nutr. 1998, 128, 251.
N.H. Romano, D. Sengupta, C. Chung, S.H. Heilshorn, Protein-engineered biomaterials: nanoscale mimics of the extracellular matrix. Biochim. Biophys. Acta 2011, 1810 339.
J. Rosenlöcher, G. Sandig, C. Kannicht, V. Blanchard, S.O. Reinke, S. Hinderlich, Recombinant glycoproteins: The impact of cell lines and culture conditions on the generation of protein species. J. Proteomics 2016, 134, 85.
E. Roth, G. Ollenschlager, G. Hamilton, A. Simmel, K. Langer, W. Fekl, R. Jakesz, Influence of two glutamine-containing dipeptides on growth of mammalian cells. In Vitro Cell Dev. Biol. 1988, 24, 696.
A. Sánchez-Kopper, M. Becker, J. Pfizenmaier, C. Kessler, A. Karau, R. Takors, Tracking dipeptides at work-uptake and intracellular fate in CHO culture. AMB Express 2016, 6, 48.
J.B. Sherwood, D. Shouval, Continuous production of erythropoietin by an established human renal carcinoma cell line: development of the cell line. Proc. Natl. Acad. Sci. USA 1986, 83, 165.
Image: 1B8M: Brain Derived Neurotrophic Factor, Neurotrophin-4
The structures of the neurotrophin 4 homodimer and the brain-derived neurotrophic factor/neurotrophin 4 heterodimer reveal a common Trk-binding site.
Robinson R.C., Radziejewski C., Spraggon G., Greenwald J., Kostura M.R., Burtnick L.D., Stuart D.I., Choe S., Jones E.Y.
Protein Sci. (1999) 8 p.2589-2597
THERAPEUTIC RECOMBINANT PROTEINS IN CLINICAL DEVELOPMENT
The development pipeline for therapeutic recombinant proteins is robust. There are over 100 recombinant proteins excluding monoclonal antibodies in active clinical development. The top therapeutic categories for recombinant proteins in development include oncology, hematology, central nervous system, endocrine, metabolic and genetic disorders, infectious diseases and cardiovascular (1).
There are numerous recombinant proteins in various phases of clinical development as shown in Figure 1. Several candidates are currently pending approval as shown in Table 1.
Figure 1: Recombinant Proteins with Highest Development in Phase I to Pending Approval (1)
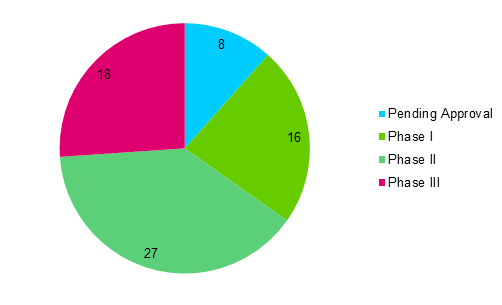
Table 1: Recombinant Proteins Pending Approval (1)
| Product Name | Active Ingredient | Condition Treated | Product Type | Companies |
|---|---|---|---|---|
| CHS1701 | pegfilgrastim | Cancer Chemotherapy Induced Neutropenia(PA) | Biosimilars | Coherus BioSciences, KBI Biopharma |
| Lifmior | etanercept | Autoimmune and Inflammatory Disorders(PA) | Biosimilars | Pfizer Inc, Pfizer Limited |
| MYL1401H | pegfilgrastim | Cancer Chemotherapy Induced Neutropenia(PA) | Biosimilars | Biocon, Mylan Inc |
| Neucardin | neuregulin 1 (recombinant, human) | Congestive Heart Failure(PA) | Investigational Drug | Zensun (Shanghai) Sci & Tech Co Ltd, TBG Diagnostics Limited, PharmaSynth, SciClone Pharmaceuticals Inc |
| TuNEX | etanercept | Ankylosing Spondylitis(PA), Juvenile Idiopathic Arthritis(PA), Plaque Psoriasis(PA), Psoriatic Arthritis(PA), Rheumatoid Arthritis(PA) | Biosimilars | Mycenax Biotech Inc, TSH Biopharm |
| Zioxtenzo | pegfilgrastim | Cancer Chemotherapy Induced Neutropenia(PA) | Biosimilars | Novartis AG, Sandoz International GmbH |
Candidates Pending Approval
CHS1701 (pegfilgrastim) is a long-acting pegylated version of the recombinant human granulocyte colony-stimulating factor (GCSF) analog known as filgrastim. Coherus Biosciences is developing CHS1701 as a pegfilgrastim (Neulasta) biosimilar candidate to treat cancer chemotherapy induced neutropenia. In 2016, Coherus submitted the biologics license application (BLA) for CHS1701 to the U.S. Food and Drug Administration (FDA). The company has also submitted a Marketing Authorization Application to the European Medicines Agency (EMA) for CHS1701 (1).
Pfizer is developing Lifmior (etanercept) for the treatment of autoimmune disorders such as arthritis and psoriasis. Lifmior is soluble dimeric fusion protein consisting of the extracellular ligand-binding region of recombinant human tumor necrosis factor (rhTNF) receptor attached to the constant (Fc) region of human immunoglobulin G (FcIgG). In 2016, the EMA recommended approval of a marketing authorization for Lifmior (1).
Biocon and Mylan are also developing a pegfilgrastim (Neulasta) biosimilar candidate for the treatment of chemotherapy induced neutropenia called MYL1401H. In 2016, the companies reported that the EMA has accepted for review, the Marketing Authorization Application for MYL1401H. In 2017, the companies reported that the FDA has accepted the BLA for MYL1401H for filing (1).
Neucardin is a recombinant peptide fragment of neuregulin-1 that is being developed by Zensun for the treatment of congestive heart failure. Zensun submitted a New Drug Application (NDA) to the China Food and Drug Administration. In 2013, the company reported that the China Food and Drug Administration said that its Phase II data is insufficient and they have asked Zensun to submit a new NDA once their Phase III study reached its endpoints (1).
Mycenax Biotech is developing an etanercept biosimilar called TuNEX for the treatment of arthritis and psoriasis. The company has completed submission to the Taiwan Food and Drug Administration for multiple indications including ankylosing spondylitis, juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis and rheumatoid arthritis (1).
Novartis is also developing a biosimilar to Neulasta (pegfilgrastim) known as Zioxtenzo. In 2017, the company withdrew its MAA for Zioxtenzo as the CHMP had doubts that the product could be approved as a biosimilar based on available data (1).
Conclusions
Recombinant proteins have become valuable therapeutic. Many companies have recombinant proteins in their development pipelines that are biosimilars or new investigational drugs. For research purposes, Bachem offers several recombinant peptides and proteins at shop.www.bachem.com.
References
(1) Medtrack (2017)
MEET BACHEM: DIVINA PERALTA
What is your official job title at Bachem?
Director of Shipping
Briefly, what do you do at Bachem?
I am responsible for managing the Shipping and Distribution Center for Bachem Americas. Our team is responsible for the distribution of GMP, catalog and custom synthesis products from the U.S.A.
How long have you been with Bachem?
I joined Bachem Americas in 2006.
Where did you work before Bachem?
Previously, I worked for a Biotech company in Torrance, CA.
What makes a perfect day for you?
A perfect day is to be able to serve our customers and meet their ever increasing demand for our products and services.
What is your business motto?
Think positive, be passionate and continue to make progress.
What do you like most about your job?
I appreciate the opportunity to interact and collaborate with our business partners and be a part of their success. My job also allows me to have the opportunity to work on projects that increase efficiencies and contribute to overall, valuable achievements.
What do you like to do outside of work (interests, hobbies)?
I enjoy taking short hikes and staying fit.
Thank you very much Divina.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Injectable solution may provide weeks of glucose control-Duke University
New approach set to make peptide stapling widely available-Science Daily
Custom built molecule shows promise as anti-cancer therapy-University of Bath
Microbe generates extraordinarily diverse array of peptides-Massachusetts Institute of Technology
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
J.K. Malet et al.
Alteration of epithelial cell lysosomal integrity induced by bacterial cholesterol-dependent cytolysins.
V.G. Martinez et al.
Neuromedin U alters bioenergetics and expands the cancer stem cell phenotype in HER2-positive breast cancer.
Int. J. Cancer 140, 2771-2784 (2017)
G. Pejler et al.
Acidic pH is essential for maintaining mast cell secretory granule homeostasis.
Cell Death Dis. 8, e2785 (2017)
M. Schmitt et al.
Peptide vaccination in the presence of adjuvants in patients after hematopoietic stem cell transplantation with CD4+ T cell reconstitution elicits consistent CD8+ T cell responses.
Theranostics 7, 1705-1718 (2017)
T.L. Tremblay and J.J. Hill
Biotin-transfer from a trifunctional crosslinker for identification of cell surface receptors of soluble protein ligands.
