The broad choice of cyclization variants leaves much room for optimizing the properties of a cyclopeptide. Numerous chemistries together with the required amino acid derivatives are at hand for ring closure.
TYPES OF CYCLES
Covalent bonds can be formed between various positions of a peptide. Most types of linkages require incorporation of non-standard amino acid derivatives. The linear precursors usually are assembled by solid-phase peptide synthesis. Partially protected linear peptides can be obtained by Fmoc-SPPS on chlorotrityl resin or Sasrin resin.
Variants of cyclization (see Fig. 1)
1) End-to-end (head-to-tail) cyclization
2) End-to-side chain cyclization (requires a trifunctional amino acid)
3) Side chain-to-side chain cyclization (requires two trifunctional amino acids. Reactive side- chains can also be generated by alkylating the amide nitrogen with a substituent bearing a second functionality)
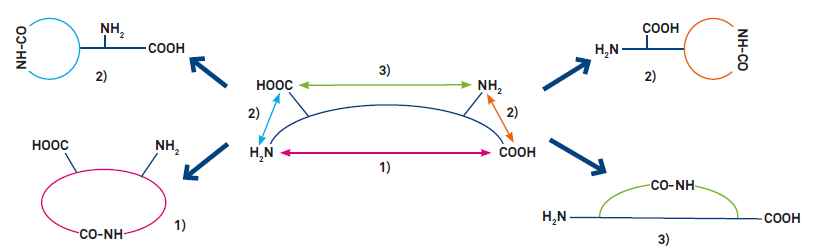
Figure 1: Variants of peptide cyclization. Most peptides can be cyclized by amide bond formation (lactamization, as shown). Modification of the termini or incorporation of suitable amino acid derivatives allows cyclization by other covalent bonds, e.g. lactone, thioether, 1,2,3-triazole
CHEMISTRIES OF CYCLIZATION
Stability, length, and rigidity of the linkage are important parameters to be evaluated, when optimizing the structure of a cyclic peptide. Irrespective of the chemistry used, ring formation is conducted under high dilution conditions as to minimize oligomerization. Another strategy circumvents the tedious work-up of high-dilution reactions: selectively cleavable protecting groups split off on-resin (often used in combination with side-chain anchoring) allow cyclizations on the carrier.
Disulfide bridges and mimics: Disulfide bridge formation is the most common variant of side chain-to-side chain cyclization and Nature’s approach for stabilizing active conformations. The bridging is reversible, as S-S bonds can be cleaved enzymatically or by reductants as DTT. Multiple disulfide bridging is often observed in bioactive peptides. When synthesizing such molecules by chemical means, the cysteine pairs can be linked simultaneously or consecutively, the latter being more laborious and considerably costlier for the customer. An array of selectively cleavable sulfhydryl protecting groups has been developed for consecutive bridging, for further details please see our e-brochure Cysteine Derivatives. Short peptides containing multiple disulfide bridges are extraordinarily stable, especially in combination with head-to-tail cyclization. Trisulfide bridges can also be generated from Cys-containing peptides. Cysteine can be replaced by sulfhydryl-containing amino acids as Pen or Hcy. Replacing terminal cysteines by cysteamine and/or mercaptoalkylcarboxylic acids markedly improves resistance to enzymatic degradation. Atosiban (H-6722), desmopressin (H-7675), and eptifibatide (H-6654, Fig. 2) may serve as examples for such an exchange.
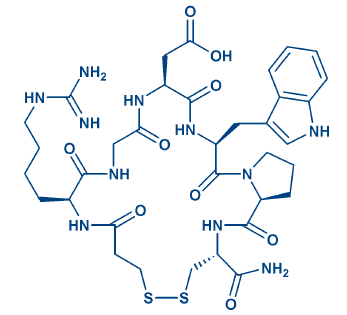
Figure 2: The RGD mimic eptifibatide.
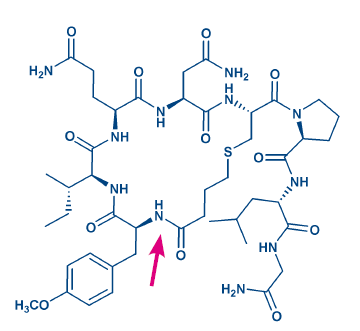
Figure 3: Structure of carbetocin. The arrow points at the site of ring formation.
Moreover, end-to-end cyclized cystine peptides can be accessed by this approach. Disulfide bonds are rather flexible, they are sensitive to strong oxidants and bases and, of course, to reductants. They may be replaced by sulfide (thioether) bonds, which can be generated by reacting the cysteine thiol with an N-terminal chloro- or bromoacetyl moiety. As the reactivity of the halogen in ω-haloalkylcarboxylic acids decreases with increasing chain length, use of the preformed thioether derivative is an attractive alternative, e.g. when synthesizing the oxytocin analog carbetocin (H-5832, Fig 3).
Replacing both sulfur atoms by aliphatic carbon yields a completely stable cystine mimic. For this end, two cysteines have to be replaced by an α-amino-α,ω-dicarboxylic acid (e.g. by incorporating Fmoc-Asu(OtBu)) or an appropriately protected α,ω-diamino-α,ω-dicarboxylic acid. In the eel calcitonin analog elcatonin (H-2214), α-aminosuberic acid (Asu) replaces the disulfide bridge of the native peptide. Insertion of an α,ω-diamino-α,ω-dicarboxylic acid can be circumvented by replacing the cysteines by ω-alkenyl-α-amino acids. Cyclization is achieved by ring-closing metathesis (RCM) followed by hydrogenation. RCM leaves a double bond, a mixture of the cis and trans isomer is formed. Application of RCM is by no means restricted to the synthesis of stapled peptides, and less sophisticated olefin derivatives such as ω-alkenoic acids can be incorporated in the linear precursor peptide.
Amide linkages: Cyclic carboxamides are also known as lactams. For head-to-tail cyclization in solution, the linear precursor has to be protected except for the terminal amino and carboxyl moieties. Ring formation involving side chains require selectively cleavable protecting groups. OAll/Aloc or highly acid-labile groups are chosen for obtaining such compounds by Fmoc-SPPS.
The ease of cyclization depends on the conformation of the peptide. A cis amide bond is required for proper folding, for generating a bend. Hence ring closing is facilitated or even only made possible by incorporation of Pro residues, N-methylamino acids, pseudoprolines, or Dmb backbone protection (such modifications will promote all types of ring closures). The beneficial effect of the incorporation of a pseudoproline moiety is shown in Fig. 4. For further information please see our e-brochure Pseudoproline Dipeptides.
The outcome of such a cyclization can be strongly influenced by the choice of coupling reagent and linkage site. So, when synthesizing a cyclic hexapeptide lacking Gly or Pro, one of the 6 amide bonds has to be chosen for ring closure. The corresponding protected linear precursor is obtained by SPPS. Besides modest cyclization yields or failure, concomitant racemization could pose a problem.
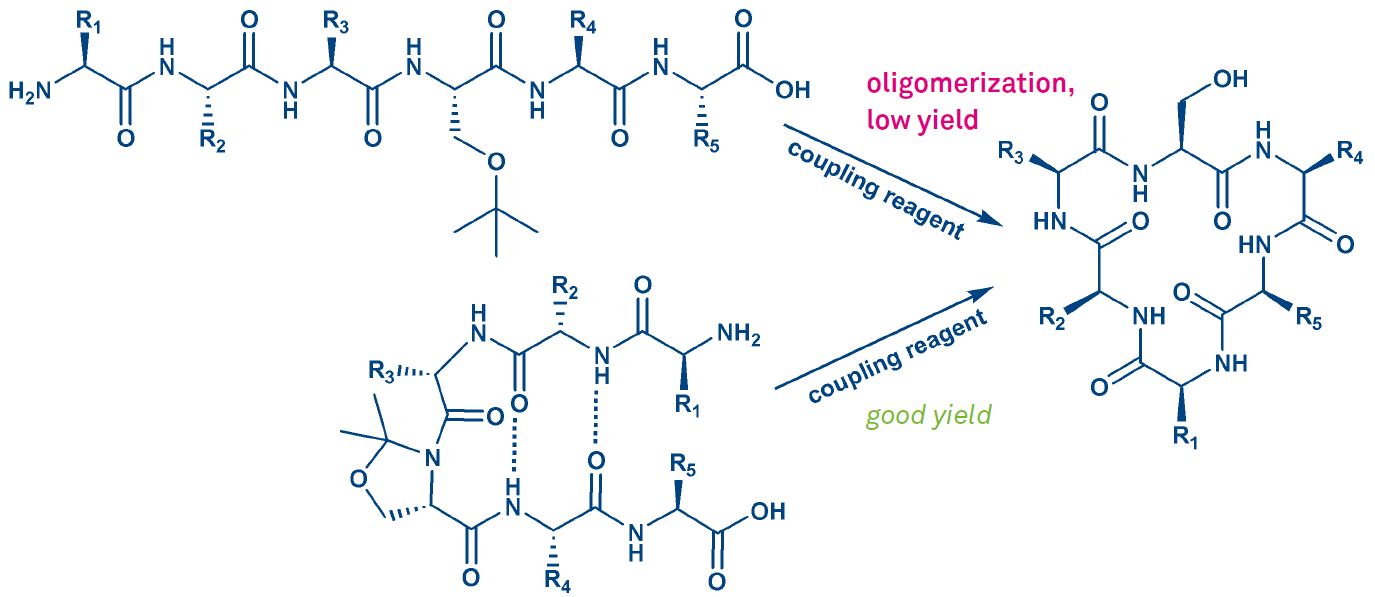
Figure 4: Cyclization of a hexapeptide employing a pseudoproline generated from serine.
Lactam Variants: End-to-end – fully protected peptide with free termini end-side chain – trifunctional amino acid: C-terminus-Lys(Aloc), Asp/Glu(OAll)-N-terminus side chain-side chain – 2 trifunctional amino acids: Lys(Aloc) and Asp or Glu allyl ester Orn, Dab, and Dab are alternatives for lysine, Asp/Glu can be replaced by the homologous Aad or Asu. Our e-brochure Orthogonality of Protecting Groups presents a compilation of suitable amino acid derivatives and combinations. Native chemical ligation offers an alternative to “standard” cyclization methods.
Other Types of Cycles: Cyclic depsipeptides contain at least one ester bond, which is formed during the penultimate synthetic step followed by deblocking. Suitably protected hydroxycarboxylic acids are required for end-to-end cyclization, whereas selective deprotection of Ser/Thr and Asp/Glu allow side chain-side chain esterification. Cyclic peptide thioesters can be obtained in the same manner. Cycles, even from completely deprotected peptides, can be generated by 1,3-dipolar cycloaddition (click chemistry) when incorporating azido and alkyne derivatives into the linear peptide, e.g. Pra and Lys(N2) for side chain ring formation, N-terminal ω-azidocarboxylic acids and C-terminal propargylamides allow end-to-end cyclization. The resulting triazole is a rigid peptide bond surrogate. Fig. 5 shows two examples. Further information can be obtained from our e-brochure Click Chemistry. RCM as well allows cyclization of unprotected peptides.
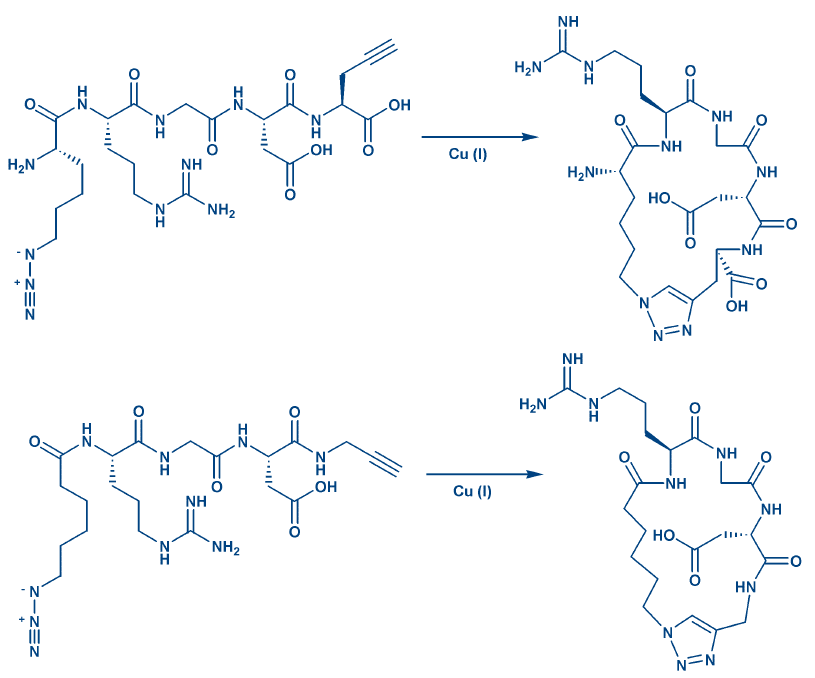
Figure 5: Side chain-side chain and end-to-end cyclization by click chemistry.
References:
Moroder, L., Besse, D., Musiol, H. J., Rudolph-Bohner, S. and Siedler, F. Oxidative folding of cystine-rich peptides vs regioselective cysteine pairing strategies, Biopolymers 40, 207-234 (1996)
Davies, J. S. The cyclization of peptides and depsipeptides, J. Pept. Sci. 9, 471 (2003)
White, C. J. and Yudin, A. K. Contemporary strategies for peptide macrocyclization, Nat. Chem. 3, 509-524 (2011)
Reinwarth, M., Nasu, D., Kolmar, H. and Avrutina, O. Chemical synthesis, backbone cyclization and oxidative folding of cystine-knot peptides: promising scaffolds for applications in drug design, Molecules 17, 12533-12552 (2012)
Roxin, A. and Zheng, G. Flexible or fixed: a comparative review of linear and cyclic cancer-targeting peptides,
Future Med. Chem. 4, 1601-1618 (2012)
Bockus, A. T., McEwen, C. M. and Lokey, R. S. Form and function in cyclic peptide natural products: a pharmacokinetic perspective, Curr Top Med Chem 13, 821-836 (2013) Thakkar, A., Trinh, T. B. and Pei, D. Global analysis of peptide cyclization efficiency, ACS Combinatorial Science 15, 120-129 (2013)
Lau, Y. H., de Andrade, P., Wu, Y. and Spring, D. R. Peptide stapling techniques based on different macrocyclisation chemistries, Chem. Soc. Rev. 44, 91-102 (2015)
Marti-Centelles, V., Pandey, M. D., Burguete, M. I. and Luis, S. V. Macrocyclization Reactions: The importance of conformational, configurational, and template-Induced preorganization, Chem. Rev. 115, 8736-8834 (2015)
Rohrbacher, F., Deniau, G., Luther, A. and Bode, J. W. Spontaneous head-to-tail cyclization of unprotected linear peptides with the KAHA ligation, Chem. Sci. 6, 4889-4896 (2015)
CYCLIC PEPTIDES IN THE PIPELINE
Naturally occurring cyclic peptides are an inspiration for peptide drug design due to their resistance to enzyme degradation and enhanced protein binding affinity. Cyclic peptides often have better biological activity compared to linear peptides due to conformational rigidity (1). In addition, many natural cyclic peptides are cell permeable.
One example is Cyclosporine A, an immunosuppressant used for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants and for the treatment of some autoimmune disorders. Cyclosporine A was approved by the FDA over thirty years ago as the product Sandimmune®. This 1200 Da natural cyclic peptide targets an intracellular protein and is orally bioavailable (2). Due to the success of cyclosporine A, cyclic peptides have long been an area of interest but natural cyclic peptides can be complex and difficult to synthesize. In recent years, this class of molecules has been propelled forward by the development of new methods to improve the drug-like properties of peptides by constraining their structure and the creation of new platforms to screen large libraries of cyclic molecules (3). Consequently, there are several cyclic peptides currently in clinical development for a variety of indications.
A selection of cyclic peptides in clinical development is highlighted in Table 1. Additional cyclic peptides are in earlier stages of development with companies such as Bicycle Therapeutics, Encycle Therapeutics, Ensemble Therapeutics, Lanthio Pharma, Tarix Pharmaceuticals, Protagonist Therapeutics and other players in this field (2).
| Product Name | Therapeutic Category | Highest Phase | Companies Involved |
|---|---|---|---|
| Aplidin® | Hematology, Oncology | III | PharmaMar SA, Specialised Therapeutics Australia Pty Ltd, TTY BioPharm |
| Debio025 | Infectious Diseases, Musculoskeletal | III | Debiopharm Group, Solid Biosciences, Novartis AG |
| MK4261 | Infectious Diseases | III | Cubist Pharmaceuticals Inc, Merck & Co Inc |
| PT141 | Genitourinary Disorders, Hematology | III | Palatin Technologies Inc, King Pharmaceuticals Inc, Gedeon Richter Plc |
| ALRN6924 | Oncology | II | Aileron Therapeutics Inc, Roche |
| APL1 | Ophthalmology, Respiratory | II | University of Pennsylvania, Alcon Laboratories Inc, Apellis Pharmaceuticals, Potentia Pharmaceuticals Inc |
| APL2 | Hematology, Ophthalmology | II | University of Pennsylvania, Apellis Pharmaceuticals, Potentia Pharmaceuticals Inc |
| ASP3291 | Gastroenterology | II | Astellas Pharma Inc, Drais Pharmaceuticals Inc |
| AT1001 | Endocrine, Metabolic and Genetic Disorders, Gastroenterology | II | Alba Therapeutics Corporation, Teva Pharmaceutical Industries Ltd, Shire plc, Cephalon Inc |
| AZP531 | Cardiovascular, Endocrine, Metabolic and Genetic Disorders | II | Alize Pharma, Eli Lilly and Company Limited |
| CIGB300 | Infectious Diseases, Oncology | II | Laboratorio Elea SACIFyA, Centro de Ingenieria Genetica y Biotecnologia |
| MEN11420 | Gastroenterology | II | The Menarini Group |
| PL3994 | Cardiovascular, Respiratory | II | Palatin Technologies Inc |
| POL6326 | Immunology and Inflammation, Cardiovascular, Oncology | II | Polyphor Ltd |
| RG7929 | Infectious Diseases | II | Polyphor Ltd, Roche |
| SCY635 | Infectious Diseases | II | SCYNEXIS Inc, Waterstone Pharmaceuticals Inc |
| Voclera™ | Dermatology, Genitourinary Disorders, Immunology and Inflammation | II | Aurinia Pharmaceuticals Inc, Paladin Labs Inc, Roche, 3SBio Inc |
| Vosoritide | Endocrine, Metabolic and Genetic Disorders | II | BioMarin Pharmaceutical Inc |
| ALRN5281 | Endocrine, Metabolic and Genetic Disorders | I | Aileron Therapeutics Inc |
| Biafungin | Infectious Diseases | I | Seachaid Pharmaceuticals, Cidara Therapeutics Inc |
| Emodepside | Infectious Diseases | I | Bayer HealthCare AG, The University of Bonn, Drugs for Neglected Diseases initiative |
| JNJ54452840 | Cardiovascular | I | Corimmun GmbH, Janssen Research & Development LLC, Janssen-Cilag GmbH |
| OBP801 | Oncology | I | Astellas Pharma Inc, Yamanouchi Pharmaceutical Co., Ltd., Oncolys, Biopharma Inc |
| RA101495 | Hematology | I | Ra Pharmaceuticals Inc |
Table 1: Cyclic Peptides in Phase I to Phase III Clinical Development (2)
Phase III Clinical Candidates:
Aplidin® (plitidepsin), a synthetic cyclic depsipeptide originally of marine origin, is under development by
PharmaMar SA. In 2015, PharmaMar reported that patient enrollment for the pivotal Phase III trial of Aplidin for
the treatment of multiple myeloma was completed (2). Aplidin is also currently in a Phase II study in relapsed or
refractory angioimmunoblastic T-cell lymphoma and a Phase Ib trial in relapsed or refractory multiple myeloma as
part of a triple combination. Aplidin has been granted orphan drug designation by the EMA and FDA (4).
Debio025 (alisporivir), a synthetic cyclosporine derivative, is a cyclophilin inhibitor with multiple potential
indications. In 2014, Novartis Pharmaceuticals completed a Phase III follow-up study to assess the viral activity in
patients who failed to achieve sustained virologic response in Debio025 studies for chronic hepatitis C patients.
Debiopharm entered into a collaboration agreement with Novartis for the development of Debio025 in 2010;
however, Debiopharm regained full rights to the alisporivir program in 2015. Later in 2015, Debiopharm and Solid
Biosciences LLC announced collaboration for preclinical studies of Debio025 in Duchenne Muscular Dystrophy (2).
Merck & Co is developing MK4261 (surotomycin), a cyclic lipopeptide with bactericidal activity. In 2015, Cubist,
the originator, completed a Phase III study of MK4261 in patients with clostridium difficile associated diarrhea.
In the same year, Cubist was acquired by Merck & Co (2).
PT141 (bremelanotide) is a cyclic peptide derivative being developed by Palatin Technologies for female sexual
dysfunction (FSD). PT141 is a synthetic analog of the naturally occurring hormone α- Melanocyte-stimulating hormone.
In 2015, Palatin announced that it completed enrollment in two pivotal Phase III studies of PT141 for the
treatment of FSD (2) Palatin anticipates that the Phase III program will last 15-18 months and if the clinical data
supports approval of PT141 for FSD, a New Drug Application will be submitted to the FDA (5).
Conclusion:
Several cyclic peptides are in advanced phases of clinical development. Cyclic peptides continue to be an enticing
class of molecules as they often exhibit improved bioactivity and in many cases overcome challenges associated
with linear peptides such as poor oral availability, membrane permeability and metabolic stability. As more peptides
of this class progress through clinical trials we will see which of the new approaches such as constrained peptides
turn into viable drug candidates. For researchers and companies engaged in this exciting area, Bachem offers a
comprehensive custom synthesis service including capabilities to produce cyclic peptides with various types of cyclization.
References:
(1) Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Sang Hoon, J. 1, s.l.: The Korean Society of Applied Pharmacology, Jan 2012, Biomol Ther (Seoul), Vol. 20, pp. 19-26.
(2) Medtrack. [Online] [Cited: February 23, 2016.]
(3) Excited about Cycling. Cain, C. September 17, 2012.
(4) Oncology Pipeline. PharmaMar. [Online] 2016. [Cited: February 25, 2016.]
https://www.pharmamar.com/science-and-innovation/oncology-pipeline/
(5) Bremelanotide for Female Sexual Dysfunction. Palatin Technologies. [Online] 2016. [Cited: February 24, 2016.]
http://www.palatin.com/products/bremelanotide.asp
