MEET US AT THE BIOFIT IN STRASBOURG

It is our pleasure to inform you that Bachem will be attending the BioFit 2017, which will take place on November 28 – 29, 2017, at the Strasbourg Convention Center, France.
BioFIT is the leading partnering event in Europe for tech transfer, academia-industry collaborations and for sourcing early-stage innovations stemming from academia, startups and SMEs of the Life Sciences field.
We look forward to networking with the BioFit participants and present our custom manufacturing capabilities for peptide APIs.
Bachem‘s pipeline contains more than 150 customer projects in preclinical and clinical phases. They all have promising potential: recently, a number of products in phase III trials received marketing authorization and phase II projects progressed to pivotal phase III clinical trials. Some of our services include pegylated peptides, lipidated peptides, various other peptide conjugates, and sterile fill and finish (Clinalfa®). An innovative service in our portfolio is the selective chemical glycosylation. The technology is applicable to large scale and has the potential to be applied to a variety of peptides, where we can pioneer the concept of improving current and future drugs. To view our webinar please click here.
We are excited to meet with our partners, learn their needs for peptides and discuss how Bachem can help to advance their peptide drug projects. We invite you to visit us at our Booth # C7: please contact us to schedule a meeting in advance.
We look forward to meeting you at the BIO-Fit 2017 in Strasbourg!
ENDOTHELINS
Importance for Medical Research
Endothelins and their receptors constitute a complex system. They are important in embryological development, have various expression patterns in tissues and organs, and are involved in numerous physiological and pathophysiological processes. The latter include pulmonary arterial hypertension (PAH), atherosclerosis, myocardial infarction, vasospasms after subarachnoid hemorrhage as well as risk of hypertension and atherosclerotic vascular disease in overweight and obese people. The ET-1 system furthermore could be an important component in the pathogenesis of atrial fibrillation (AF), a disease which basically resembles an abnormal heart rhythm.
Endothelins and their receptors are involved in the control of kidney functions such as renal blood flow, water and sodium excretion, and acid-base balance. Obviously, they also play a role in numerous cancer-relevant processes. ET-1 might influence the growth and progression of a variety of tumors such as prostatic, ovarian, colorectal and brain tumors as well as cervical carcinomas and melanomas.
Pain signaling is thought to occur by binding of ET-1 to ET-A receptors, localized on nociceptors. Therefore, ET-A antagonists could serve as pain relieving agents in many acute diseases, but also for patients suffering chronic pain. ET-1 also enhances the expression of adhesion molecules on vascular endothelial cells and stimulates the aggregation of polymorphonuclear neutrophils, contributing to inflammation and endothelial dysfunction.
Other diseases, for which the endothelin system is of high medical interest, are preeclampsia, a disease for which effective therapeutic options are still not available, Hirschsprung’s disease, a multigenic developmental disorder, and eventually sepsis.
Endothelins
The endothelins are a family of 21-amino acid vasoconstrictor peptides comprising three isoforms, endothelin-1 (ET-1), endothelin-2 (ET-2), and endothelin-3 (ET-3). They mediate their actions via two receptors, ET-A and ET-B, found in several mammalian species. ET-1 is the most potent mammalian vasoconstrictor identified to date with EC50 values in the subnanomolar range. ET-2 and ET-3 differ from ET-1 in two and six amino acids, respectively. The members of the endothelin family contain four cysteine residues, which form two intra-chain disulfide bridges linking cysteine residues 1 to 15 and 3 to 11.
The major source of ET-1 are endothelial cells, but it is also produced by epithelial cells, macrophages, fibroblasts, cardiac myocytes, and neurons. It is likely to have a role in controlling perfusion in every organ in the body. ET-1 is synthesized in a constitutive pathway for continuous release and in a second pathway involving most probably degranulation from special storage granules upon external stimulus. ET-2 is expressed in intestinal epithelial cells and at lower levels in the heart. ET-3 is expressed in brain neurons, kidney, and intestinal epithelial cells. The endothelins show very close structural and functional relationship to the snake venom peptides sarafotoxins (Figure), which comprise four isoforms, S6A, S6B, S6C and S6D (or SRTX-A, -B, -C and –D).
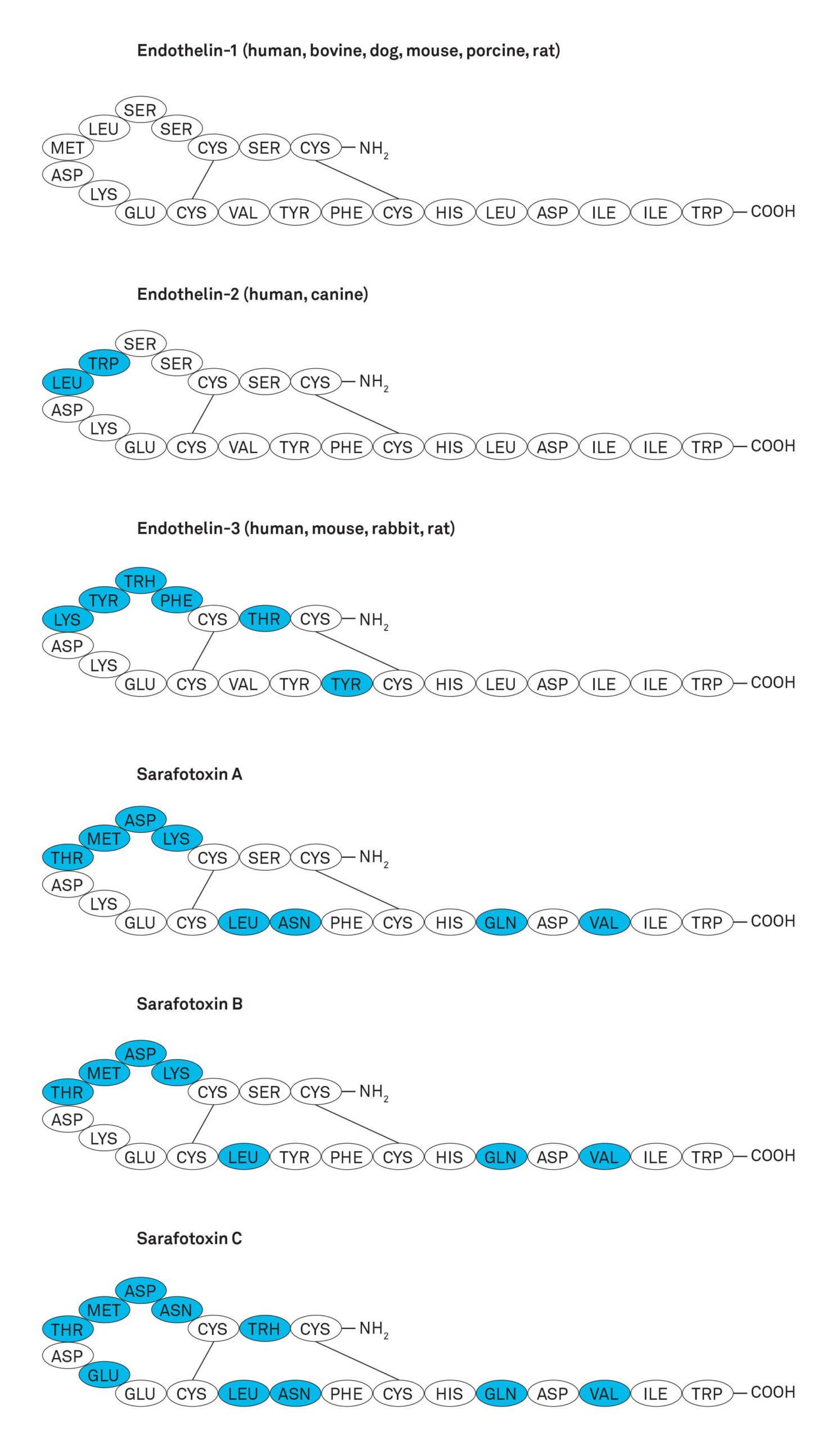
Figure: Structure of endothelins and sarafotoxins. Amino acids different to the primary sequence of ET-1 are shown in blue color.
Processing, Regulation and Clearance
Mature endothelins are created by sequential proteolytic processing of preproendothelins. Removal of the signal peptide and further processing results in the production of physiological inactive precursors, the big endothelins. These are further cleaved by endothelin converting enzymes (ECE) to form active endothelins. ECE are type II membrane proteins with two known members, ECE-1 and ECE-2, and various isoforms (ECE-1 A-D and ECE-2 A-C). The synthesis and secretion of ET-1 is increased by various growth factors, cytokines and vasoactive factors. Hypoxia and shear stress have also been shown to increase ET-1 release from endothelial cells. ET-1 expression is suppressed by several factors including atrial natriuretic peptide, nitric oxide, prostacyclin, and heparin. ET-1 peptide is rapidly cleared from the plasma with a half-life of several minutes.
Endothelin Receptors
ET-A and ET-B belong to the superfamily of the seven transmembrane G-protein coupled receptors (GPCRs) with an extracellular N-terminal and an intracellular C-terminal domain. ET-A and ET-B receptors have contrasting functions under physiological conditions. The stimulation of ET-A receptors in the smooth muscle leads to constriction, whereas activation of the ET-B receptors in the endothelial cells leads to the release of vasodilators.
ET-1 and ET-2 have similar binding affinities for the ET-A, but differ slightly in their affinities for ET-B. ET-3 preferentially binds to ET-B receptor and hence can be considered as moderately selective for ET-B. The affinity of ET-1 to both, ET-A and ET-B resembles an autocrine feedback mechanism, since both receptors are counter-acting. This mechanism presumably is important for the cardiovascular homeostasis. Obviously, low levels of ET-1 promote vasodilatation, whereas higher and pathophysiological concentrations increase blood pressure and total peripheral vascular resistance.
Modified endothelins and peptides acting on endothelin receptors in general are indispensable tools to investigate the processes and mechanisms associated with these fascinating GPCRs.
Please explore our broad offering of Endothelin Research Peptides and Sarafotoxins
References
ENDOTHELIN RECEPTOR ANTAGONISTS AND AGONISTS IN CLINICAL DEVELOPMENT
The endothelin A and endothelin B receptors mediate the actions of endothelin and both receptor subtypes are targets for developing therapies for human diseases. Three endothelin-receptor antagonists, bosentan, ambrisentan and macitentan, are already approved for the treatment of pulmonary arterial hypertension (PAH) (1). In the future, endothelin receptor antagonists and agonists might find a place in the treatment of other diseases such as diabetic nephropathy, cerebral vasospasm, cancer and diabetic ketoacidosis. There are many endothelin receptor antagonists in various phases of clinical development as shown in Table 1.
| Product Name | Active Ingredient | Condition Treated | Highest Phase | Companies |
|---|---|---|---|---|
| Xinlay | atrasentan hydrochloride | Diabetic Nephropathy(III) | Pending Approval (Current Phase III) | Abbott Laboratories, AbbVie Inc |
| Pivlaz | clazosentan | Cerebral Aneurysm(II) | Phase III | Roche, Vernalis plc, Idorsia Pharmaceuticals Ltd, Axovan, Ltd., Actelion Pharmaceuticals Ltd |
| RE021 | sparsentan | Glomerular Disorders(III), Diabetic Nephropathy(II), Hypertension(II), Immunoglobulin A Nephropathy(II) | Phase III | Bristol-Myers Squibb Company, Retrophin Inc, Pharmacopeia, Inc., Ligand Pharmaceuticals Inc |
| ACT132577 | despropyl macitentan | Hypertension(II) | Phase II | Actelion Pharmaceuticals Ltd, Johnson & Johnson, Janssen Biotech Inc, Idorsia Pharmaceuticals Ltd |
| SPI1620 | -- | Solid Tumors(I) | Phase II (Current Phase I) | University of Illinois at Chicago, Chicago Labs Inc, EndogenX Inc, Spectrum Pharmaceuticals |
| PMZ2123 | -- | Diabetic Ketoacidosis(I) | Phase I | Pharmazz Inc |
Phase III Candidates
Xinlay (atrasentan hydrochloride), a pyrrolidine derivative, is an endothelin A receptor antagonist that AbbVie is developing for the treatment of diabetic nephropathy. Abbott Laboratories was developing Xinlay for the treatment of prostate cancer but the U.S. Food and Drug Administration rejected the company’s New Drug Application for the prostate cancer indication back in 2005 (2). Development continued for the diabetic neuropathy indication and in 2013, AbbVie initiated a Phase III clinical study to assess the effects of atrasentan on renal outcomes in patients with type 2 diabetic nephropathy while they continue to be treated with the current standard of care (1).
Actelion Pharmaceuticals is developing Pivlaz (clazosentan) for the reversal of vasopasm associated with aneurysmal subarachnoid hemorrhage. In 2017, Acetlion completed a Phase II study to assess the efficacy and safety of clazosentan in reversing cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage treated by surgical clipping or endovascular coiling (1).
Retrophin is developing RE021 (sparsentan), a dual-acting endothelin and angiotensin receptor antagonist. Sparsentan is being developed for the treatment of diabetic nephropathy, a rare kidney disorder known as focal segmental glomerulosclerosis (FSGS), immunoglobulin A nephropathy and hypertension. Sparsentan has orphan drug designation from the U.S. Food and Drug Administration and European Commission for FSGS (3). The product is in a Phase II/III clinical trial for treatment of FSGS (1).
Phase II Candidates
Idorisa Pharmaceuticals, a spin-off from Actelion Pharmaceuticals, is developing ACT132577 (aprocitentan) as a treatment for cardiovascular disorders such as resistant hypertension. ACT132577 is a dual antagonist of the endothelin A and endothelin B receptors (1). The company completed Phase II clinical trials and is working on the design of a Phase III program that will consist of two studies evaluating the effect of ACT-132577 on systolic and diastolic blood pressure in patients with true resistant hypertension (4).
Phase I Candidates
SPI1620 is a peptide agonist of endothelin B receptors expressed on endothelial cells lining the interior surface of blood vessels. Spectrum Pharmaceuticals is developing SPI1620 as an adjunct therapy in solid tumors. The peptide works by stimulating endothelin B receptors and modifying blood flow to increase delivery of anticancer agents to the tumor (5). In 2012, Spectrum completed a Phase I study of SPI1620 in patients with recurrent or progressive carcinoma (1).
Pharmazz also has an endothelin receptor antagonist, PMZ2123, in Phase I clinical trials. PMZ2123, also known as BQ 123, is a cyclic peptide in development for the treatment of cerebral edema in diabetic ketoacidosis (1). In addition, the company recently received an exclusive license that will allow Pharmazz to develop PMZ2123 for the treatment of opioid tolerance and pain management (6).
Conclusion
Clinical trials are exploring new applications of endothelin A and endothelin B receptor antagonists and agonists. To support organizations and studying the endothelin system, Bachem offers a selection of endothelin and related peptides available at shop.www.bachem.com. In addition, Bachem offers custom peptide synthesis, production of peptide-based new chemical entities and generic active pharmaceuticals ingredients.
References
(1) Medtrack (2017)
(2) FDA reject Abbott’s prostate cancer drug Xinlay, PharmaTimes (2017)
(3) Sparsentan, Retrophin (2017)
(4) Actelion provides an update on the progress towards launching Idorsia, GlobeNewswire (2017)
(5) SPI-1620, Spectrum Pharmaceuticals (2017)
(6) Pharmazz, Inc. has received exclusive license to U.S. patent for treatment of opioid tolerance and pain management, PR Newswire (2017)
MEET BACHEM: GABRIEL LAUBER
What is your official job title at Bachem?
I am working as an Inside Sales Representative.
How long have you been with Bachem? Where did you work before Bachem?
I entered my position on the 15th of August 2017. Before I found my way to Bachem, I completed an apprenticeship as a merchandiser at AudioRent Clair in Aesch.
Briefly, what do you do at Bachem?
Currently I work as a Sales Representative for research-grade materials. We serve customers from academia and research institutes with our expertise and a portfolio of over 6500 research products.
What do you like to do outside of work (interests, hobbies)?
In my spare time, I am a passionate semiprofessional singer and songwriter. My Artist name is Gabriel Benedek. I play piano, guitar and I produce my own Trap/Pop music together with a friend. Our biggest achievement so far: We have 3 singles which are daily played by national and local radio stations. What I also enjoy a lot is to go running, doing fitness sports and martial arts tricking. I enjoy reading good books, love cooking and being with friends.
What do you like most about your job?
Most of all I like my team and my daily tasks as they are very interesting and the work is exciting. I love the contact with our global clients and hope I will get the chance to connect to different clients around the world even more.
What is your business motto?
A satisfied customer is the best business strategy of all.
Thank you very much Gabriel.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Major advance in nanopore detection of peptides and proteins-University of Groningen
Imaging agents developed to better monitor growth of tumours-University of Alberta
Scientists develop broad-spectrum inhibitors of influenza viruses-The Scripps Research Institute
Pill for glycemic control for type 2 diabetes shows promise-EurekAlert!
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
R. Cianfrocca et al.
Blocking endothelin-1-receptor/beta-catenin circuit sensitizes to chemotherapy in colorectal cancer.
Cell Death Differ. 24, 1811-1820 (2017)
R.R. Guddeti et al.
Role of endothelin in microvascular dysfunction following percutaneous coronary intervention for non-ST elevation acute coronary syndromes: a single-centre randomised controlled trial.
C.C. Lien et al.
Chronic endothelin-1 infusion causes adipocyte hyperplasia in rats.
Obesity (Silver Spring) 24, 643-653 (2016)
S. Gulati et al.
Attenuation of opioid tolerance by ETB receptor agonist, IRL-1620, is independent of an accompanied decrease in nerve growth factor in mice.
