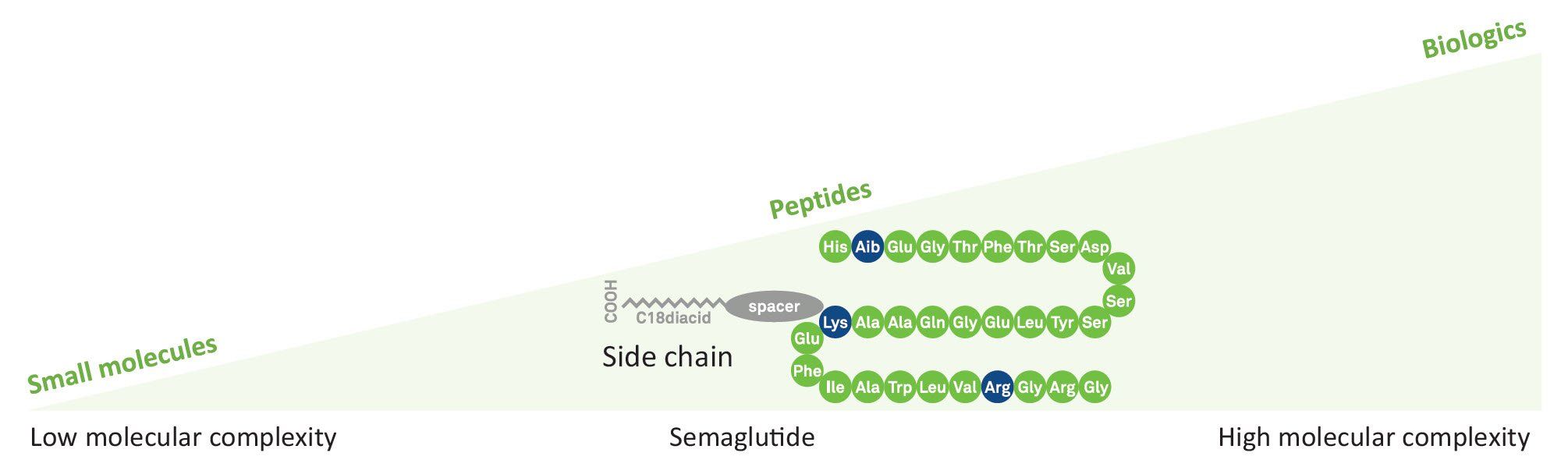
SEMAGLUTIDE
What is Semaglutide?
Semaglutide is a lipopeptide with a linear sequence of 31 amino acids. Like human Glucagon-like peptide-1 (GLP-1), it is used in combination with diet and exercise in the therapy of type 2 diabetes mellitus. It works as an anti-obesity agent, a neuroprotective agent, and an appetite depressant.
Semaglutide can be commercialized as a synthetic generic product after approval of an abbreviated new drug application (ANDA) or comparable applications with other regulatory authorities. The length and modifications make technical excellence and regulatory expertise a prerequisite for efficient filing and fast approval. High material quality and yield, robust processes including secure supply and innovative approaches at high-tech facilities will enable our customers to achieve their business goals.
ENSURING YOU A SMOOTH REGISTRATION PROCESS
Bachem has a long-standing track record in with successful registrations of highly purified synthetic peptide drugs of the glucagon family. Our regulatory intelligence keeps track of important changes in the relevant legislation. This enables us to be a leading global innovator in the field of glucagon and glucagon-like synthetic peptide drugs. Our services have been optimized to shorten timelines and of reduce complexity for our clients.
scalability, innovation and automation
From small to large-scale production with high quality
Our high-tech Good Manufacturing Practice (GMP) facilities based in Switzerland and the US, plus the commitment of our technical and scientific experts to quality, are the cornerstones for continuous compliance. We deliver small-scale to multi-kg active pharmaceutical ingredients (APIs) with impurities below 0.5%, identification and characterization of impurities above 0.10% using orthogonal analytical techniques.
Optimized, automated high-yield production
Our long experience in complex APIs allows us to optimize the processes for high yields at outstanding quality. Our high level of process automation allows for cost-efficient and large-scale production resulting in excellent overall material purity (>99.5%). Innovative solutions like continuous chromatography let us use equipment and resources more efficiently and help us and our partners to achieve their commitment to sustainability and environment-friendly production. Our GMP sites in Switzerland and California comply with and surpass the most stringent regulations.
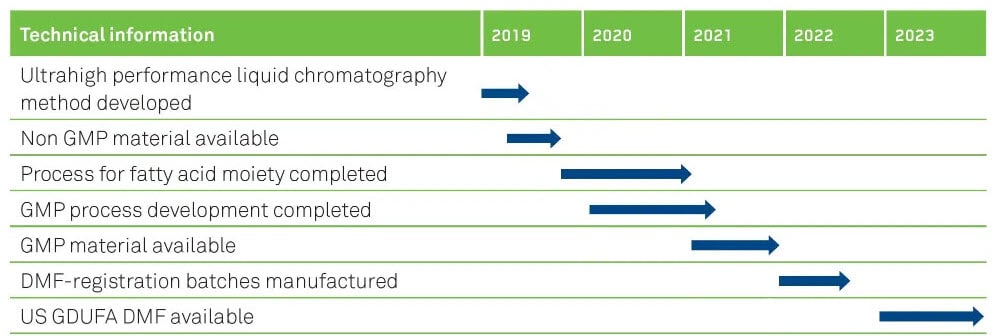
OUR TIMELINES FOR SEMAGLUTIDE
Robust processes and supply security
Having our in-house building blocks for peptide synthesis as well as long-term cooperation with trusted suppliers ensures on-time production. Redundancy of multi-purpose equipment and facilities helps to mitigate risks in the supply chain, together with our stock of finished APIs and is the key for on-time product deliveries to our customers.
services
Semaglutide impurities
The success of an abbreviated new drug application depends largely on the impurity profile of the synthetic peptide drug compared to the impurity profile of the reference listed drug (RLD) and the level of achieved “sameness”. Bachem identifies impurities, characterizes peptide-related impurities that are above 0.10% of the drug substance or greater, and provides impurities as catalog products.
Related products (for research purposes only)
Beyond GMP-grade semaglutide, we provide semaglutide in research grade, variants thereof and in different salt forms.
Related APIs
Bachem also provides exenatide, liraglutide, glucagon and other APIs for diabetes management.
Bachem Regulatory Documentation
US GDUFA DMF anticipated to be available early in 2023
Synonyms
910463-68-2
NN9535
Ozempic
Rybelsus
NN 9535
NNC 0113-0217
NN-9535
Semaglutide [USAN:INN]
Wegovy
Rybelsus (oral semaglutide)
GTPL9724
Ozempic (injectable semaglutide)
CHEBI:167574
EX-A2424
AC-32580
NNC-0113-0217
Rybelsus;Ozempic;NN9535;OG217SC;NNC 0113-0217
Sémaglutide
Sequence
H-His-Aib-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Val-Ser-Ser-Tyr-Leu-Glu-Gly-Gln-Ala-Ala-Lys(AEEAc-AEEAc-γ-Glu-carboxyheptadecanoyl)-Glu-Phe-Ile-Ala-Trp-Leu-Val-Arg-Gly-Arg-Gly-OH
Fields of Application
Treatment of type II diabetes mellitus
Treatment of obesity
Active Substance
The peptide API Semaglutide acts in the same way as GLP-1, the hormone produced in the gut. It helps increase the amount of insulin released by the pancreas in response to consumed food. Semaglutide allows patients with type 2 diabetes to control their blood sugar levels better.
Molecular Information
Molecular Formula
C187H291N45O59
Relative Molecular Mass
4113.64 g/mol
CAS-Number
910463-68-2
Long-term Storage
-20 ± 5°C
Contact US
