MEET US AT THE 7TH INDIAN PEPTIDE SYMPOSIUM

Join us at the 7th Indian Peptide Symposium to be held at Hyderabad on February 28th to March 1st, 2019. The symposium is organized by the Indian Peptide Society and will take place at the Birla Institute of Technology and Science (BITS) Pilani, Hyderabad Campus, Shameerpet, Hyderabad.
The meeting will focus on the chemistry and structure of peptides, novel endogenous peptides, combinatorial chemistry, peptide protein interactions, structure activity relationships, molecular modelling of peptides, and peptides in genomics, proteomics, peptide drug discovery and analytical techniques in peptide research.
Bachem supports its customers in the pursuit of groundbreaking discoveries that further scientific advances, particularly in the field of medicine and diagnostics. A comprehensive catalog of biochemicals deliverable ex-stock, an exclusive custom syntheses service for research labs and a full range of services to the pharma and biotech industries complete our service portfolio.
Our colleagues Martina Diekmann (Vice President Global Marketing) and Stefanie Dobitz (Sales Manager Custom Synthesis) are excited to meet with you at the Bachem booth, learn your needs for peptides and discuss how Bachem can assist to advance your research. Please contact us to schedule a meeting in advance.
We look forward to meeting you at the 7th Indian Peptide Symposium in Hyderabad!
DIAGNOSTIC PEPTIDES

A fast and accurate diagnosis of a disease is the first step in its successful treatment. Ideally, the disease is quickly identified directly in the healthcare facility. While in some cases faster diagnostic methods will require confirmation by laboratory tests, this so-called point-of-care diagnosis is in the focus of current clinical developments, because it has several benefits.
- It increases the chance of fighting the disease at an earlier stage and therefore shortening its duration.
- It reduces the risk of trial-and-error treatment and overprescription.
- It allows for a more specialized therapy (e.g. avoiding broad-spectrum antibiotics).
- It decreases the risk of nosocomial infections by faster quarantining of infected patients. A striking example of this is the Ebola epidemic in Sierra Leone of 2014-2015. Estimates suggest that its scale could have been reduced by up to 30%, if rapid testing methods had been available.
Peptides play an important role in the detection of diseases and can be grouped into two categories based on the role they play in the diagnosis.
The first category contains biomarkers. The concentration of these peptides and/or their degradation products in body fluids change in a disease-indicating manner. You can find an overview of the biomarker peptides offered by Bachem in our product flyer. The determination of these concentrations usually requires a well-equipped laboratory and the transport of the samples, which is detrimental to a fast diagnosis.
The second group of peptides are epitopes of pathogens. These offer the potential for rapid diagnosis by detecting antibodies, which have been produced by the human body as a response to the antigens.
The standard method for detecting antibodies using diagnostic peptides is an enzyme-linked immunosorbent assay (ELISA). Although the exact method can differ from case to case, the general procedure is as follows
- Peptide sequences specific to the antibody of interest are linked to the walls of a microplate, in which the residual binding sites are blocked afterwards.
- The microplate is washed with the analyte, causing present antibodies to bind to the bound peptides.
- The microplate is washed with a mild detergent, removing any unbound biomolecules.
- The microplate is tested for the presence of bound antibodies (e.g. using an anti-antibody tagged with a fluorophore), which would indicate the presence of a disease.
The final state of a successful antibody detection is summarized in Figure 1.
Figure 1: Schematic representation of an antibody detected via ELISA (cropped, source).
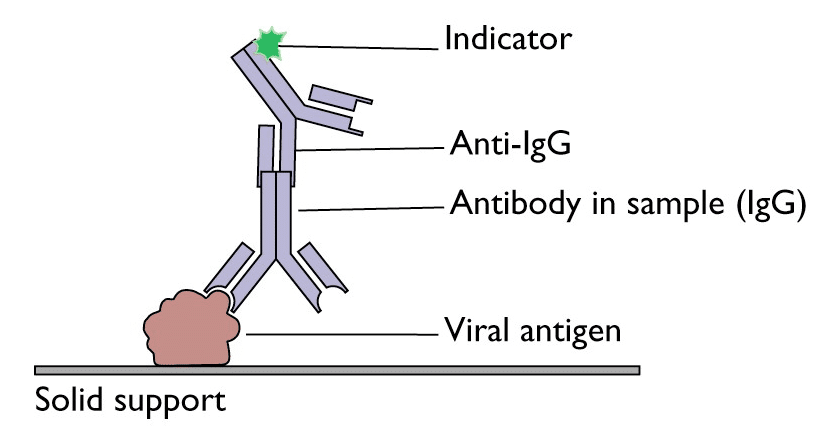
Usually the plates can afterwards be washed with a washing solution, which removes the antibodies (e.g. containing 2-mercaptoethanol), allowing the same plate to be used several times.
This methodology has been known since the 1970s. However, developments of the last 20 years have enabled a much faster discovery of new potentially useful epitopes, such as:
- The structural elucidation of complete pathogenic proteins, made possible by advances in proteomics
- The availability of standardized microwell plates, which provide a rapid, high-throughput platform for immunological testing of epitopes
The large amount of epitope data, which is available nowadays, requires fast and efficient approaches to find the best candidates.
One way to determine which part of a pathogen sequence binds to a specific antibody is to test a set of short (10-18 AAs) overlapping peptide sequences for binding to the antibody. This method allows the detection of linear antibody binding epitopes. For complex pathogens, this of course requires a very large library of synthetic peptides, which is why in these cases bioinformatics tools are used to determine epitope regions of interest.
The use of short peptide sequences offers the advantage of easier synthetic access but does unfortunately come with drawbacks. Many epitopes are either non-linear or influenced by the three-dimensional confirmation of the peptide chain. In this case, it might be possible to detect binding epitopes using longer peptides (up to 30 AAs) for the screening or again by using bioinformatics tools.
The challenge in designing diagnostic peptides is that one needs to find sequences that not only bind strongly to the antibody of interest (high sensitivity), but also only to this antibody (high selectivity). Binding to other antibodies will lead to cross-reactivity, which in turn leads to false positive results and potentially treatment of the wrong disease. Another example of problematic interference is the detection of tuberculosis (TB) antibodies in patients who are also HIV positive. Due to the alteration of the normal host immune response in persons infected with HIV, antibody tests for TB can return false negative results, since the B-cell relevant B-cell epitopes differ in comparison to HIV negative patients. This leads to a potentially deadly delay in treatment and the risk of infection of other patients. The case of TB in conjunction with HIV is especially problematic since in low-income countries both diseases are prevalent.
It is therefore necessary to check candidate sequences against such cross-reactivity to ensure the accuracy of the test even in case of multiple simultaneous diseases. This is another area where bioinformatics plays an increasingly important role. With a given sequence and epitope databases such as the IEDB algorithms can predict antibodies with the potential of cross-reactivity with this sequence, which can then be tested by an ELISA.
Despite all these obstacles, antibody detection has emerged as an important diagnostic tool. Examples of successfully established procedures are the rapid plasma reagin test (RDR) for syphilis as well as the rapid HIV-test. Both tests do however require follow-up tests for confirmation in the case of positive results.
An important factor for the reproducibility of antibody detection tests is the purity of the peptide that is used. Synthetic peptides are at an advantage in this regard compared to natural or recombinant biomolecules/pathogens, which can also be used to detect antibodies. Synthetic peptides can be produced in a highly reproducible manner, which is crucial for the commercialization of a diagnostic method using peptides. Bachem offers a custom synthesis service, which is ideally suited for our partners in the diagnostic business.
Our Center of Excellence for Custom Synthesis in St. Helens (UK) is certified according to ISO 13485 for the manufacture of peptides as raw materials for medical devices. It offers a state of the art quality control system as well as additional services such as dedicated equipment and change control.
References
S.J.Carmona, P.A.Sartor, M.S.Leguizamón, O.E.Campetella, F.Agüero, Diagnostic peptide discovery: prioritization of pathogen diagnostic markers using multiple features. PLOS ONE 2012, 7, e50748.
R.H.Meloen, W.C.Puijk, J.P.M.Langeveld, J.P.M.Langedijk, P.Timmerman, Design of synthetic peptides for diagnostics. Current Protein & Peptide Science 2003, 4, 253-260.
K.A.Navalkar, S.A.Johnston, P.Stafford, Peptide based diagnostics: Are random-sequence peptides more useful than tiling proteome sequences? Journal of Immunological Methods 2015, 417, 10-21.
P.Nouvellet, T.Garske, H.L.Mills, G.Nedjati-Gilani, W.Hinsley, I.M.Blake et al, The role of rapid diagnostics in managing Ebola epidemics. Nature 2015, 528, S109.
G.Shen, D.Behera, M.Bhalla, A.Nadas, S.Laal, Peptide-based antibody detection for tuberculosis diagnosis. Clinical and Vaccine Immunology 2009, 16, 49-54.
R.E.Soria-Guerra, R.Nieto-Gomez, D.O.Govea-Alonso, S.Rosales-Mendoza, An overview of bioinformatics tools for epitope prediction: Implications on vaccine development. Journal of Biomedical Informatics 2015, 53, 405-414.
PEPTIDES IN CLINICAL DEVELOPMENT FOR DIAGNOSTICS AND IMAGING
Clinical imaging aims to improve the diagnosis, prognosis and treatment of patients. New optical medical imaging technologies offer highly sensitive, non-invasive methods for the real-time visualization and monitoring of biological processes at a molecular level. In particular, fluorescently labeled peptides have become attractive tools for image-guided surgery and diagnostics in cancer and other disease areas. Fluorescent peptide-based probes are designed with a sequence that is able to bind selectively or become modified by the molecular target that is overexpressed at the disease site. There are several peptides in clinical development for use in diagnostics and imaging including those shown in Table 1.
| Product Name | Generic Name | Indication | Highest Phase | Companies |
|---|---|---|---|---|
| BLZ-100 | tozuleristide | Prostate Cancer; Lung Cancer, Colorectal Cancer; Pediatric CNS tumors | III | Blaze Bioscience |
| AVB620 | -- | Breast Cancer | II | Avelas Biosciences |
| EMI-137 | -- | Colorectal Cancer | II | Edinburgh Molecular Imaging Ltd. |
| LUM015 | -- | Colorectal Cancer; Pancreatic Cancer, Esophageal Cancer; Breast Cancer | II | Lumicell |
| Neutrophil Activation Probe | -- | Lung Disease | II | University of Edinburgh |
| FIB ONE | -- | Fibrosis; Lung Cancer | I | University of Edinburgh |
Phase III
Blaze Bioscience is developing BLZ-100, a tumor paint, for use in cancer surgery. BLZ-100 consists of a probe derived from chlorotoxin, which has the ability to bind selectively to several types of cancer cells and a fluorescent dye which emits light in the near-infrared range. This specialized imaging technology allows surgeons to distinguish between tumor and normal tissue during surgery. BLZ-100 is currently in a Phase II/III study in pediatric patients with central nervous system (CNS) tumors undergoing surgery (1).
Phase II
Avelas Biosciences is developing AVB620 as an in vivo surgical diagnostic agent for breast cancer. AVB620 is a fluorescent protease-activated peptide that detects, marks and diagnoses cancer. AVB620 is administered before surgery and imaging is performed with a fluorescence imaging camera. The technology is designed to enable surgeons to identify critical cancer margins and stage lymph nodes. In June 2017, Avelas initiated a Phase II study of AVB620 in breast cancer (2).
Edinburgh Molecular Imaging is developing EMI-137 as a high affinity optical probe for detecting colon cancer. EMI-137 is a sulfonated Cy5-tagged 26 amino acid peptide containing two disulfide bridges. The peptide targets c-Met, a protein associated with tumor growth that is overexpressed in colorectal cancer cells and cancer precursor cells. EMI-137 is currently in a Phase II study for colorectal cancer (3).
Lumicell is developing an imaging system to guide surgical removal of cancerous tissue. The system utilizes LUM015, a cathepsin-activatable fluorescent probe. The probe consists of the Cy5 fluorophore linked via a pan-cathepsin protease cleavable peptide, to a fluorescent quencher. The peptide is cleaved by cathepsins overexpressed by tumor cells, which releases the quencher and activates the fluorophore (4). The company has completed a Phase I trial of LUM015 in breast cancer and sarcoma. LUM015 is currently in a Phase II trial for the detection of gastrointestinal cancers (5).
Researchers at the University of Edinburgh are developing Neutrophil Activation Probe (NAP) to detect activated neutrophils in real-time in the alveolar region of patients. NAP is a dendrimeric compound that is delivered to the alveolar region of a patient in microdoses (≤100 micrograms). It becomes fluorescent only on contact with activated neutrophils and can be detected by optical endomicroscopy. NAP is currently undergoing a clinical study in Adult Respiratory Distress Syndrome (ARDS) in intensive care (3).
Phase I Candidates
Researchers at University of Edinburgh are also developing a peptide-based matrix metalloproteinase (MMP) probe, called FIB ONE, for the detection of fibrosis in the lung. This probe consists of a fluorophore linked to a quencher by a peptide sequence that is selectively cleavable by one or more MMP. FIB ONE is currently in a Phase I study to assess whether the imaging probe can detect fibrotic activity in the lung (6).
Conclusion
Novel peptide-based probes are playing a key part in the development of diagnostic and imaging tools for applications in cancer and other diseases. To support the development of peptide-based probes for research and clinical applications, Bachem provides a one-stop shop for the labeling of peptides. In addition, Bachem offers a comprehensive custom peptide synthesis service and the production of New Chemical Entities.
References
(1) Study of Tozuleristide and the Canvas Imaging System in Pediatric Subjects with CNS Tumors Undergoing Surgery. ClinicalTrials.gov (2018)
(2) AVB-620. Avelas Biosciences (2019)
(3) M. Staderini, et al., Peptides for Optical Medical Imaging and Steps Towards Therapy. Bioorg. Med. Chem. 26(10), 2816-2826 (2018)
(4) NCI Drug Dictionary. National Cancer Institute (2019)
(5) Clinical Trials. Lumicell (2019)
(6) Imaging FIB ONE in the Human Lung Using Endomicroscopy (FIB ONE). ClinicalTrials.gov (2015)
MEET BACHEM: CLAUDIA MURAR
What is your official job title at Bachem?
I work as Sales Manager Generics at Bachem.
How long have you been with Bachem? Where did you work before Bachem?
I am working at Bachem since August 2018. Previously I was a Pioneer Fellow at ETH Zurich and worked as an entrepreneur towards building a start-up on cancer immunotherapy.
What is your academic background?
I have a BSc and MSc in organic chemistry, and in 2017 I completed my doctoral studies in peptide and protein chemistry at the ETH Zurich.
What do you like most about your job?
I like the fact that every day is different. I enjoy its dynamics and the interactive exchanges. I love the combination between science, technical background and business. My job covers so many aspects and includes internal communication within different departments as well as external communication to the customers.
What do you like to do outside of work?
I love traveling and nature. I enjoy hiking during the summer and outdoor activities and sports.
Thank you very much Claudia.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Peptide isolated from spirulina extract may counteract arterial hypertension-News Medical Life Sciences
Vampire bat venom could hold key to new medical treatments-Science Daily
Using machine learning to design peptides-Northwestern University
Study identifies clinical risks and biomarkers that could be used to screen patients with heart condition-University of Birmingham
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
P.S.Filippou et al.
Biochemical characterization of human tissue kallikrein 15 and examination of its potential role in cancer.
Clinical Biochemistry 58, 108-115 (2018)
M.L.Lara-Marquez et al.
Threshold-stimulated kallikrein activity distinguishes bradykinin- from histamine-mediated angioedema.
Clinical & Experimental Allergy 48, 1429-1438 (2018)
V.Serafín et al.
An electrochemical immunosensor for brain natriuretic peptide prepared with screen-printed carbon electrodes nanostructured with gold nanoparticles grafted through aryl diazonium salt chemistry.
Talanta 179, 131-138 (2018)
X.Zhong et al.
On-chip spyhole nanoelectrospray Ionization mass spectrometry for sensitive biomarker detection in small volumes.
Journal of The American Society for Mass Spectrometry 29, 1538-1545 (2018)
