This scientific poster was originally presented in 2009.
C. Stähelin1), F. Dick1), S. Ferrari1) and D. Rentsch2)
1)Bachem AG, Bubendorf – Switzerland 2)Swiss Federal Laboratories for Materials Testing and Research (EMPA), Dübendorf – Switzerland
Introduction
The use of high quality starting materials in SPPS is a prerequisite to obtain peptides of high purity and with high yields.1T. Vorherr, F. Dick, M. Mergler, B. Sax, J. Schwindling, J. Vizzavona and P. Weiler; Proceedings of the 7th Chinese Peptide Symposium 2002, 236-240; eds: Y.-C- Du, Y.-S. Zhang and J. P. Tam. Beside AA derivatives and reagents, the quality of employed resins like Fmoc-AA-Wang is also of crucial importance. Due to the insolubility of resins, the range of analytical methods suitable to assess their quality directly is restricted.
Many attempts have been described in literature to overcome this diffi culty2B. Yan; Analytical Methods in Combinatorial Chemistry; Technomic Publishing 20003R.C. Anderson, J.P. Stokes and M.J. Shapiro; Tetrahedron Lett.1995, 36, 5311-53144B.J. Egner and M. Bradley; Drug Discovery Today 1997, 2; 102-109. Nevertheless, the quality control of resin beads still remains challenging compared to building blocks and reagents. MAS NMR enables direct analysis of resin beads without prior cleavage and sample preparations. 1D 13C MAS NMR with conventional MAS probes has already been used successfully to determine quantitatively the loading of resins using internal references5R. Hany, D. Rentsch, B. Dhanapal, D. Obrecht; J. Comb. Chem. 2001, 3, 85-89.
The significantly better resolution of HR-MAS probes enabled quantitative results from 1D 1H MAS NMR spectra as well6J. Blas, A. Rivera-Sagredo, R. Ferritto, J. F. Espinosa; Magn. Reson. Chem. 2004, 42 950-9547L. H. Lucas, M. A. Cerny, Y. M. Koen, R. P. Hanzlik, C. K. Larive; Anal. Bioanal. Chem. 2004, 380, 627-631.. Owing to overlap of resonances, the 1D NMR methods cannot always be successfully applied, particularly in cases with weak resonances. Herein, results obtained with the much more powerful HSQC tool on the generic example Fmoc-Ala-Wang resin are presented.
Results and Discussion
Wang resin (1.3 mmol/g) was loaded with a substoichiometric amount of Fmoc-Ala-OH via the standard esterifi cation method (DCCI/DMAP in DMF/THF at 0°C), aiming at an incomplete loading. The resulting Fmoc-Ala-Wang resin (0.43 mmol/g, corresponding to approx. 33% of the theoretical full loading) was subsequently treated with benzoylchloride/pyridine in THF to cap the remaining active sites leading to a 33:67 mixture of Fmoc-Ala-Wang resin and benzoyl-Wang resin.
This resin shows characteristic signals in the 13C MAS NMR spectrum which can be assigned to the corresponding structures (Fig.1). Characteristic signals of Fmoc-Ala-Wang can be identifi ed at 172.2 ppm (C-(11)), 155.0 ppm (C-(14)), 66.4 ppm (C-(10)), 49.1 ppm (C-(12)) and 18.0 ppm (C-(13)). As a result of endcapping the residual hydroxyl groups of Wang to benzoyl-Wang, a second signal of carboxyl C-(11) can be observed at 165.8 ppm.
The integral ratio of the Fmoc-Ala-Wang signals C-(11), (12), (13) and (14) to C-(11) of benzoyl-Wang enables to determine quantitatively the proportions of Fmoc-Ala- and benzoyl-Wang. For the resin shown in Fig. 1, a content of 34% Fmoc-Ala-Wang versus 66% benzoyl-Wang has been determined which corresponds well with the substitution determined by Fmoc cleavage / UV absorption.
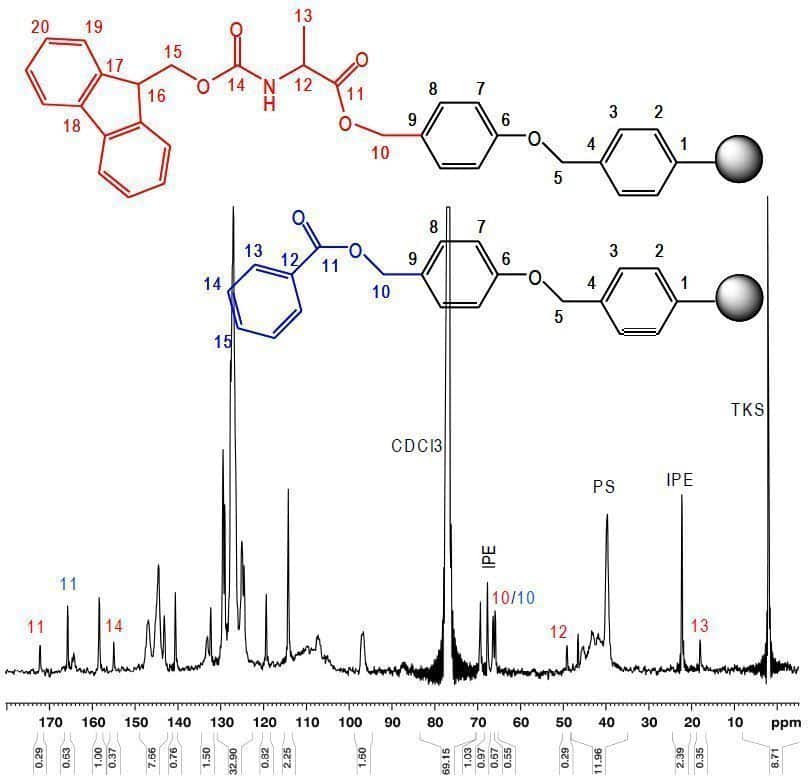
Fig. 1: Structure and 1D 13C MAS NMR spectrum of Fmoc-Ala-Wang resin with benzoyl endcapped residual Wang sites recorded at 100.6 MHz (Bruker Avance 400, MAS rate 2 kHz) with assignment of characteristic signals.
Potentially, the characteristic signal at 64.1 ppm enables to determine quantitatively the content of residual unreacted hydroxyl groups in Fmoc- AA-Wang resins. However 10% Wang resin or more had to be spiked to Fmoc-Ala-Wang in order to receive a Wang-C-(10) signal using 1D 13C MAS NMR, even at recording times of 12 hours (Fig. 2c).
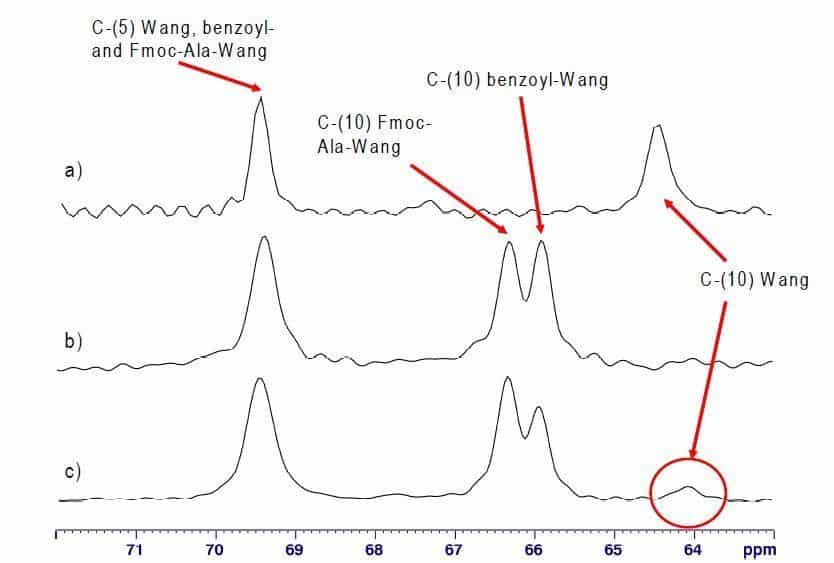
Fig. 2: Expansions of 13C MAS NMR spectra of a) Wang resin, b) benzoyl endcapped Fmoc-Ala-Wang resin and c) benzoyl endcapped Fmoc-Ala-Wang resin spiked with 10% Wang (100.6 MHz, Bruker Avance 400, MAS rate 2 kHz).
A significantly lower LOD of unreacted Wang hydroxyl groups can be achieved by 1H,13C-HSQC HR-MAS NMR. The 2D spectrum of Fmoc- Ala-Wang spiked with 10% Wang resin shows a characteristic cross signal of H-(10) / C-(10) at 4.7/64.1 ppm (Fig. 3). The same Fmoc-Ala- Wang lot spiked with only 2% Wang resin still shows a clear cross signal (Fig. 4). The intensity of the cross signal depends directly on the amount of Wang resin spiked. Quantitative determinations using calibration curves are therefore possible.
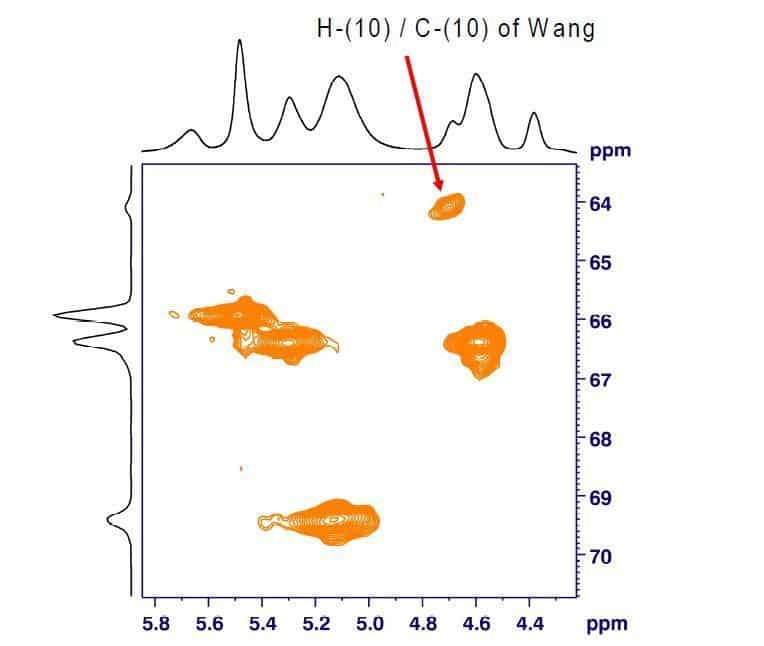
Fig. 3: Expansion of the 1H,13C-HSQC HR-MAS NMR spectrum of benzoyl endcapped Fmoc-Ala-Wang spiked with 10% Wang (Bruker Avance 400, MAS rate 4 kHz).
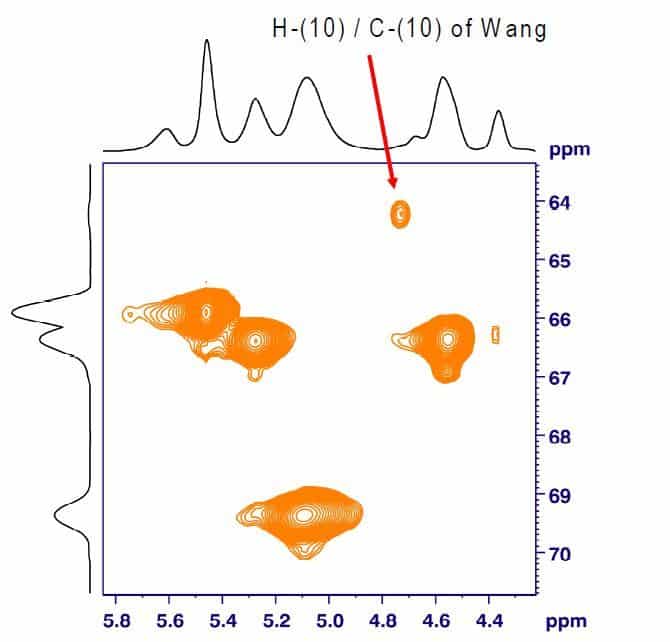
Fig. 4: Expansion of the 1H,13C-HSQC HR-MAS NMR spectrum of benzoyl endcapped Fmoc-Ala-Wang spiked with 2% Wang (Bruker Avance 400, MAS rate 4 kHz).
Conclusion and Outlook
Abbreviations
AA amino acid
DCCI 1,3-dicyclohexylcarbodiimide
DMAP 4-(N,N-dimethylamino)pyridine
DMF N,N-dimethylformamide
HR high resolution
HSQC heteronuclear single quantum coherence
IPE diisopropylether
LOD limit of detection
MAS magic angle spinning
SPPS solid phase peptide synthesis
THF tetrahydrofuran
TKS tetrakis(trimethylsilyl)silane
Acknowledgement
The authors thank D. Moskau (Bruker BioSpin AG, Switzerland) for enabling the 1H,13C-HSQC HR-MAS NMR measurements.
Subscribe to our newsletter
"*" indicates required fields
- 1T. Vorherr, F. Dick, M. Mergler, B. Sax, J. Schwindling, J. Vizzavona and P. Weiler; Proceedings of the 7th Chinese Peptide Symposium 2002, 236-240; eds: Y.-C- Du, Y.-S. Zhang and J. P. Tam
- 2B. Yan; Analytical Methods in Combinatorial Chemistry; Technomic Publishing 2000
- 3R.C. Anderson, J.P. Stokes and M.J. Shapiro; Tetrahedron Lett.1995, 36, 5311-5314
- 4B.J. Egner and M. Bradley; Drug Discovery Today 1997, 2; 102-109
- 5R. Hany, D. Rentsch, B. Dhanapal, D. Obrecht; J. Comb. Chem. 2001, 3, 85-89
- 6J. Blas, A. Rivera-Sagredo, R. Ferritto, J. F. Espinosa; Magn. Reson. Chem. 2004, 42 950-954
- 7L. H. Lucas, M. A. Cerny, Y. M. Koen, R. P. Hanzlik, C. K. Larive; Anal. Bioanal. Chem. 2004, 380, 627-631.