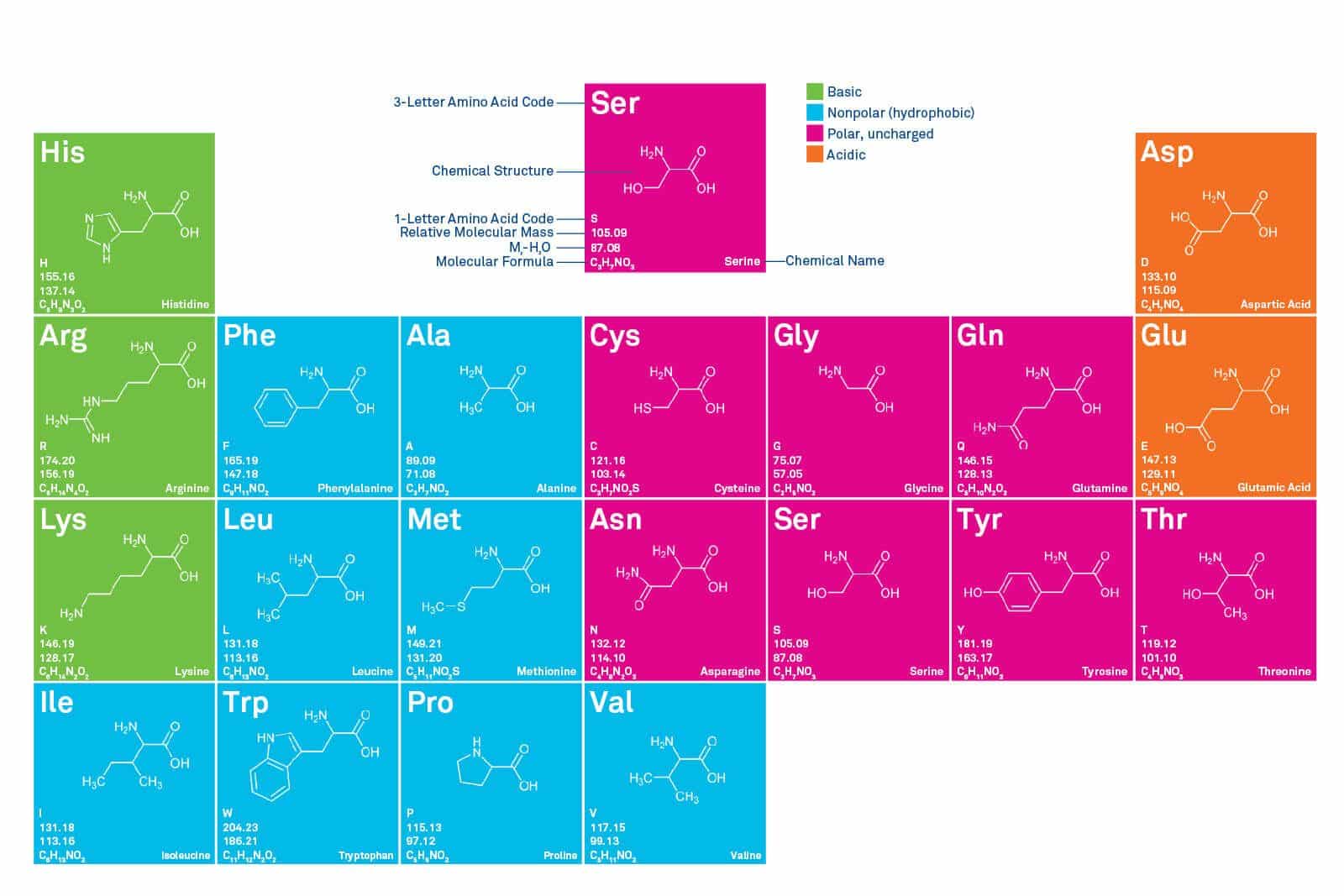
Chart of Amino Acids and its 20 proteinogenic amino acids
There are acidic, basic, polar and non-polar amino acids. They are distinguished by different colors in the “Periodic Chart of Amino Acids ” which is pictured above and can be downloaded below.
An organism such as the human body must have all 20 proteinogenic amino acids available in order to synthesize proteins. They all need to be in the L-form (except Gly). A distinction is drawn between essential and non-essential amino acids. Non-essential amino acids can be synthesized by the body itself; essential cannot, i.e. they must be supplied in the diet.
The properties of peptides and proteins are determined by the side chains of the amino acids from which they are constructed. Amino acids can also be subdivided according to the side chain that determines their properties.
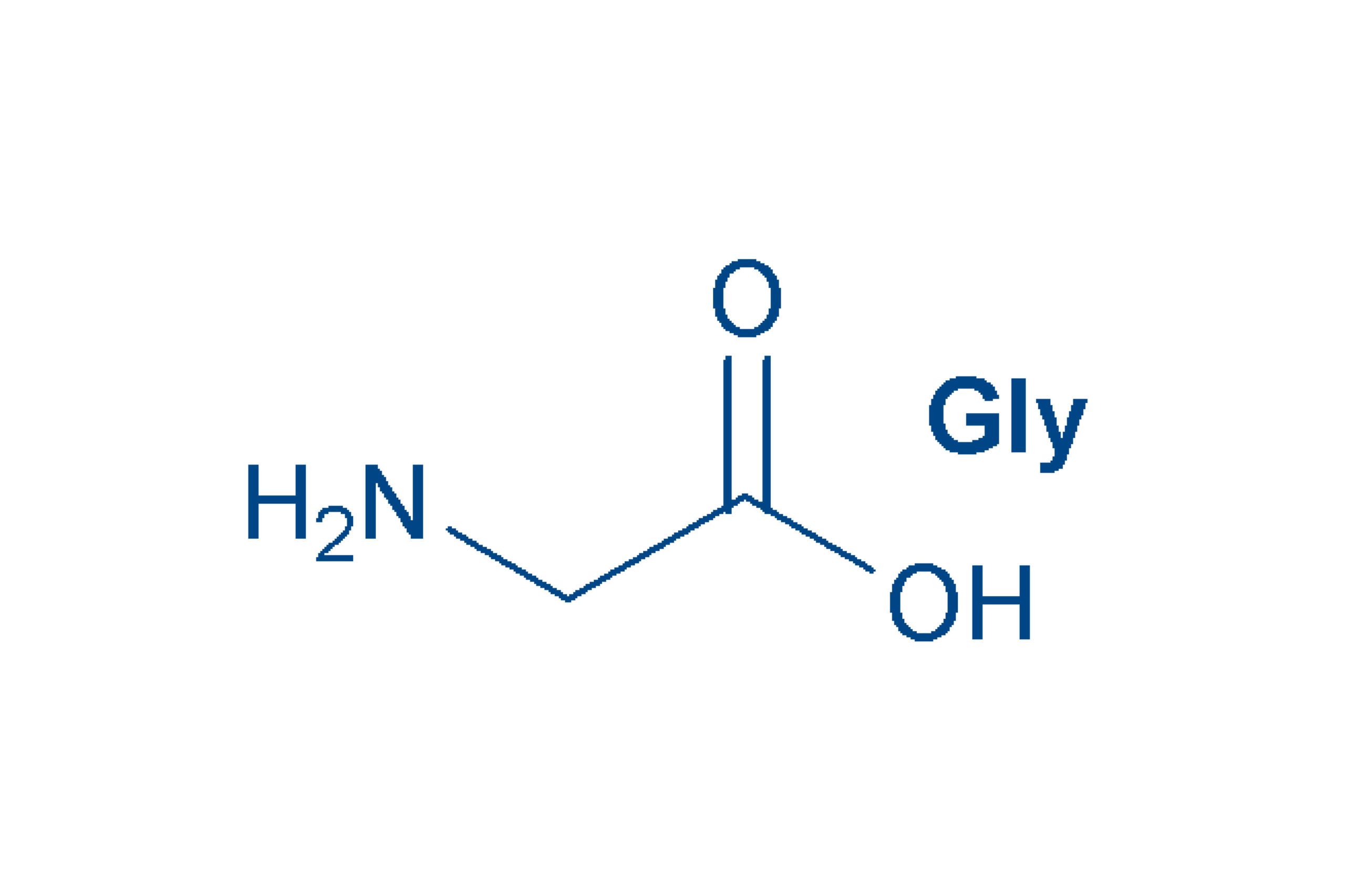
Glycine (Gly, G)
is the simplest amino acid and one of the most frequently encountered in peptides/proteins. Gly is non-essential. Glycine owes its name to its sweet taste.
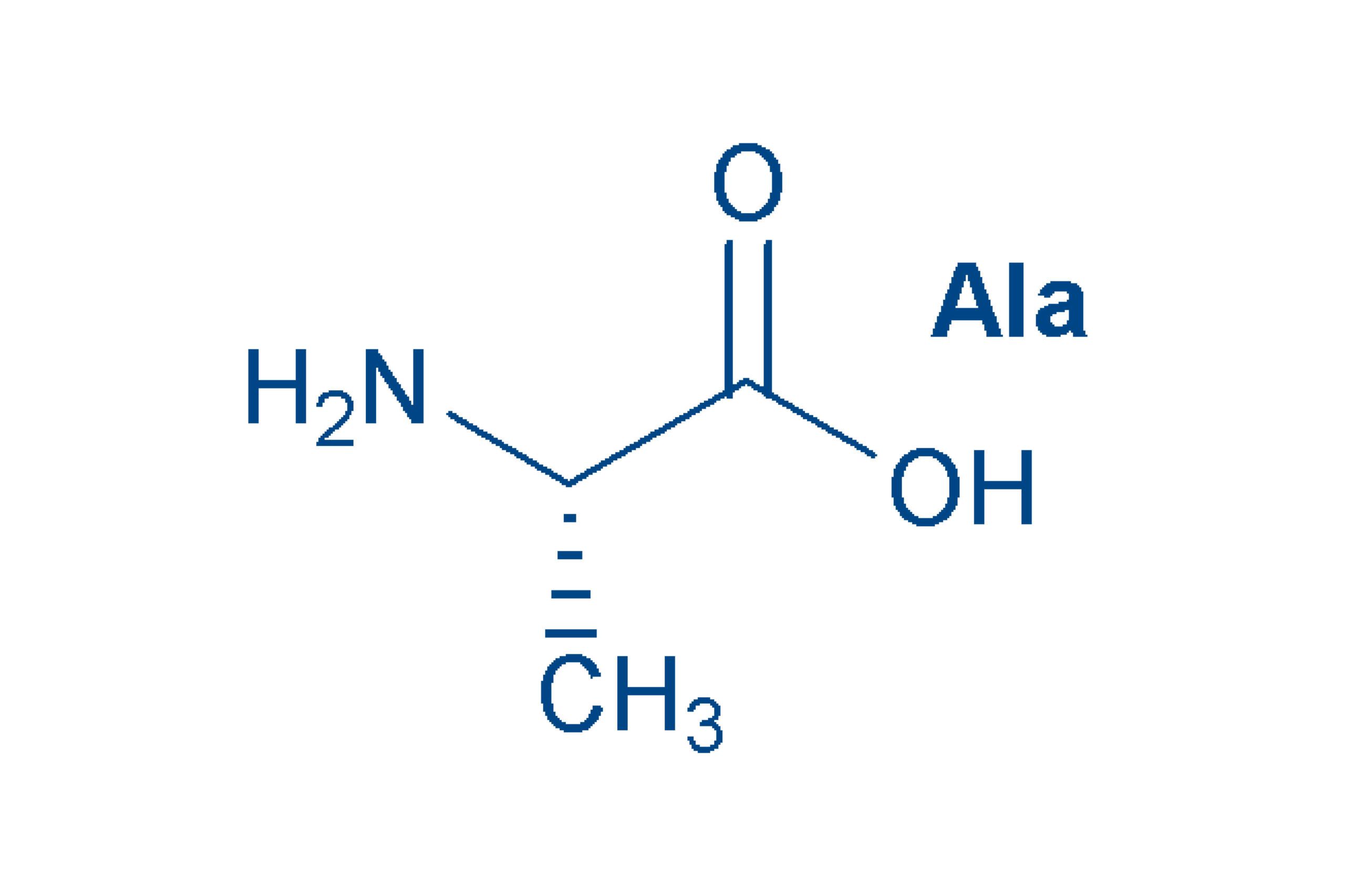
Alanine (Ala, A)
is the simplest optically active amino acid and the second most common in proteins. The L-form shown here of this non-essential amino acid is incorporated into proteins by the body, but D-alanine is also found quite frequently in nature in peptides. Alanine is one of the non-polar amino acids.
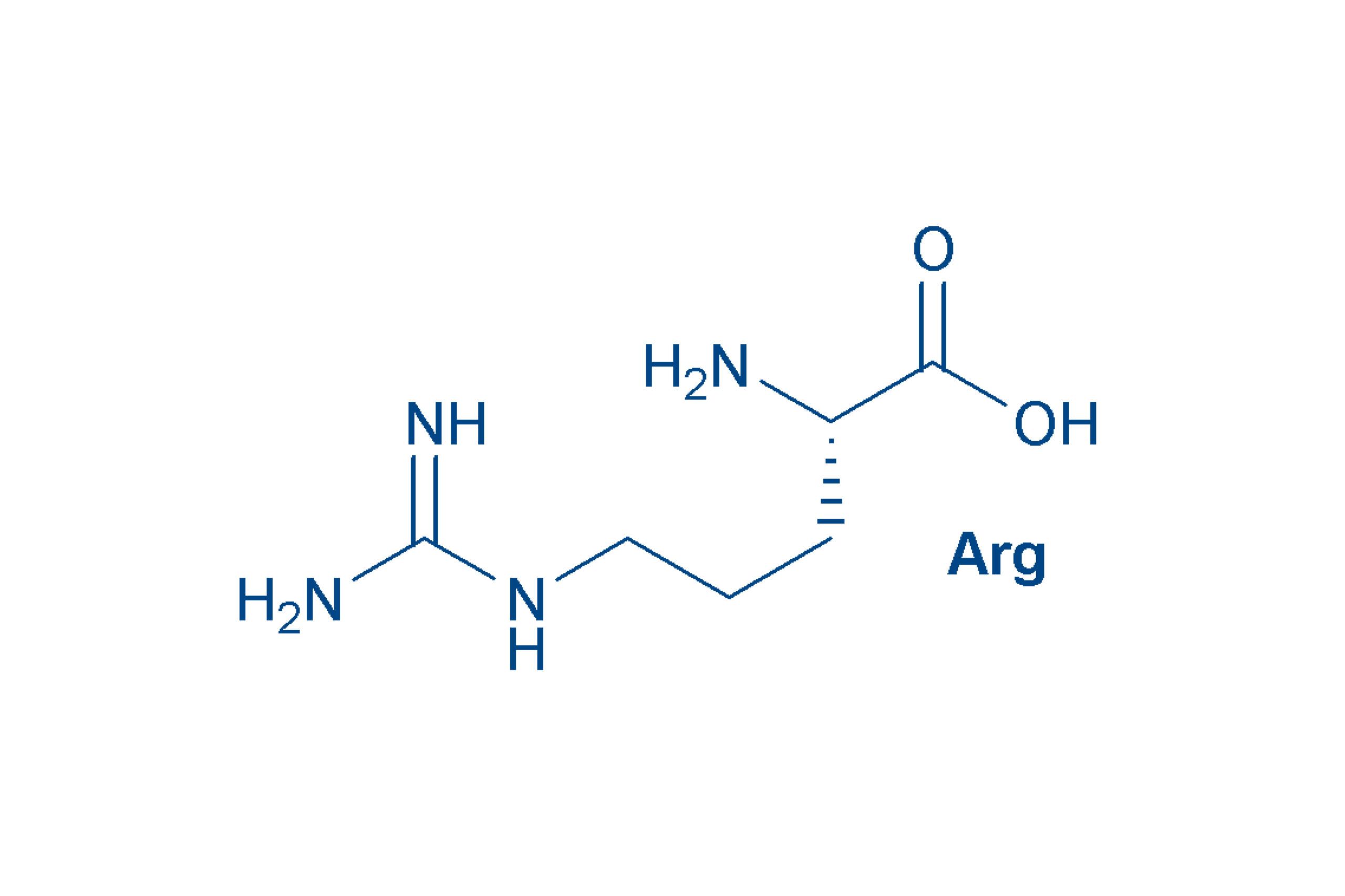
Arginine (Arg, R)
is a strongly basic amino acid. The nitrogen-containing guanidino group in the side chain is such a strong base that it forms a salt with virtually any acid. It owes its name to the fact that it was isolated as the silver salt. Arg is semi-essential (the body can produce it, but often in insufficient amounts). Arg-containing peptides are highly polar and readily water-soluble.
Aspartic acid (Asp, D)
is an acidic amino acid because it contains a further acid group in the side chain. This persists when Asp is incorporated into a peptide. Aspartic acid was discovered in asparagus, which helped give it its name. Asp is non-essential for mammals.
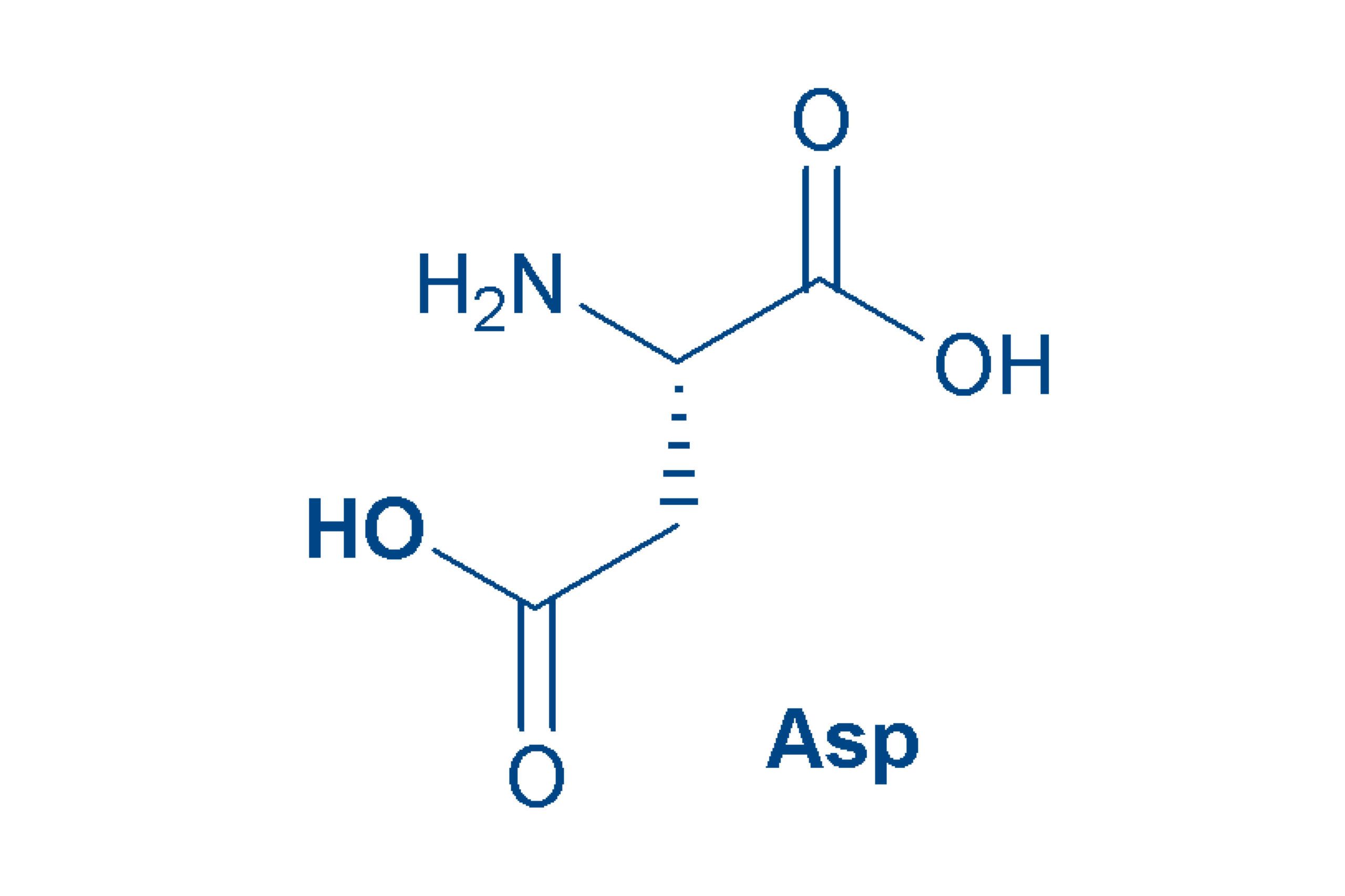
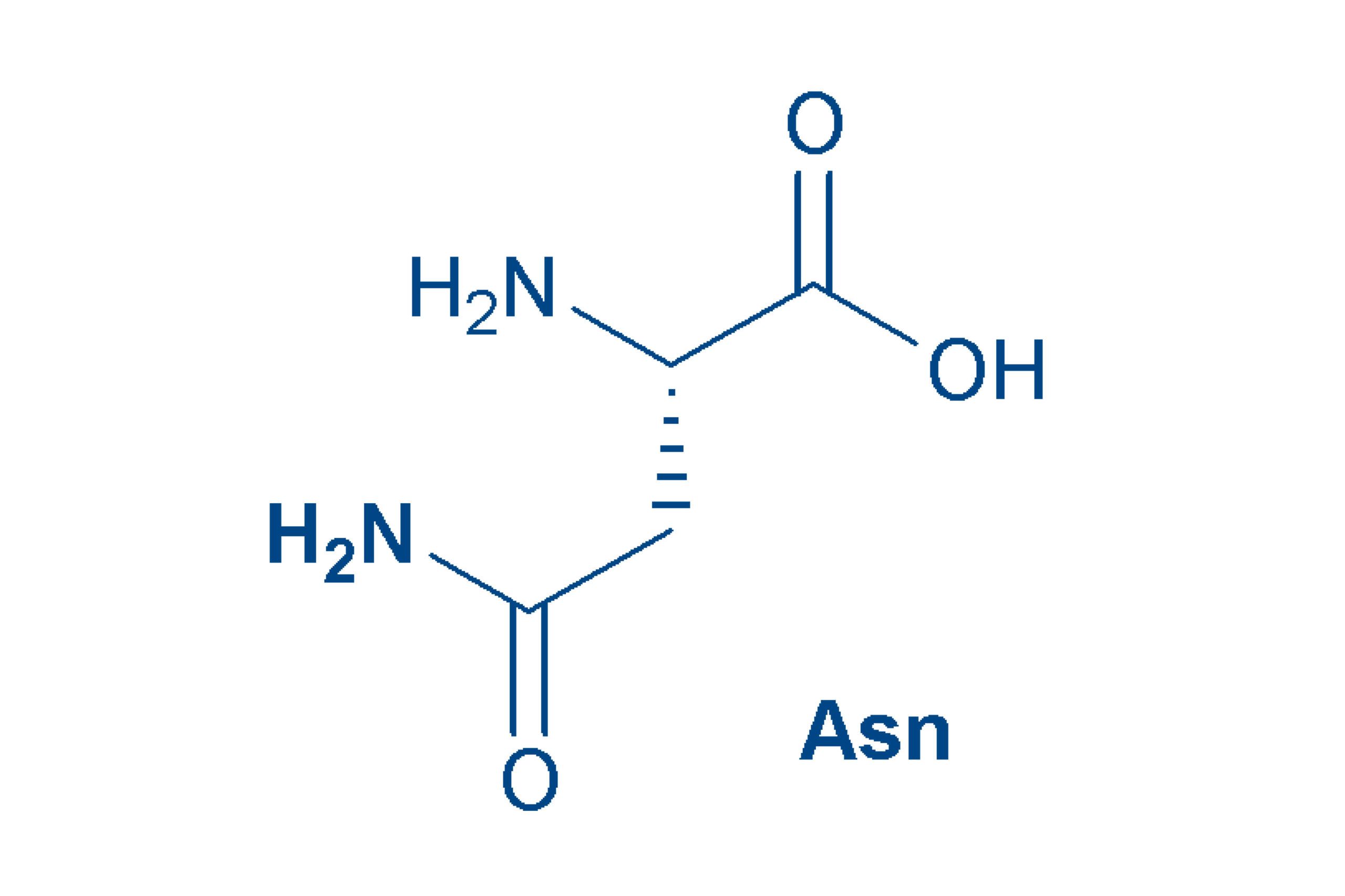
Asparagine (ASN, N)
is actually a derivative of aspartic acid (the amide) and like the latter, was discovered in asparagus. Asn is a polar amino acid and is relatively labile in peptides and proteins.
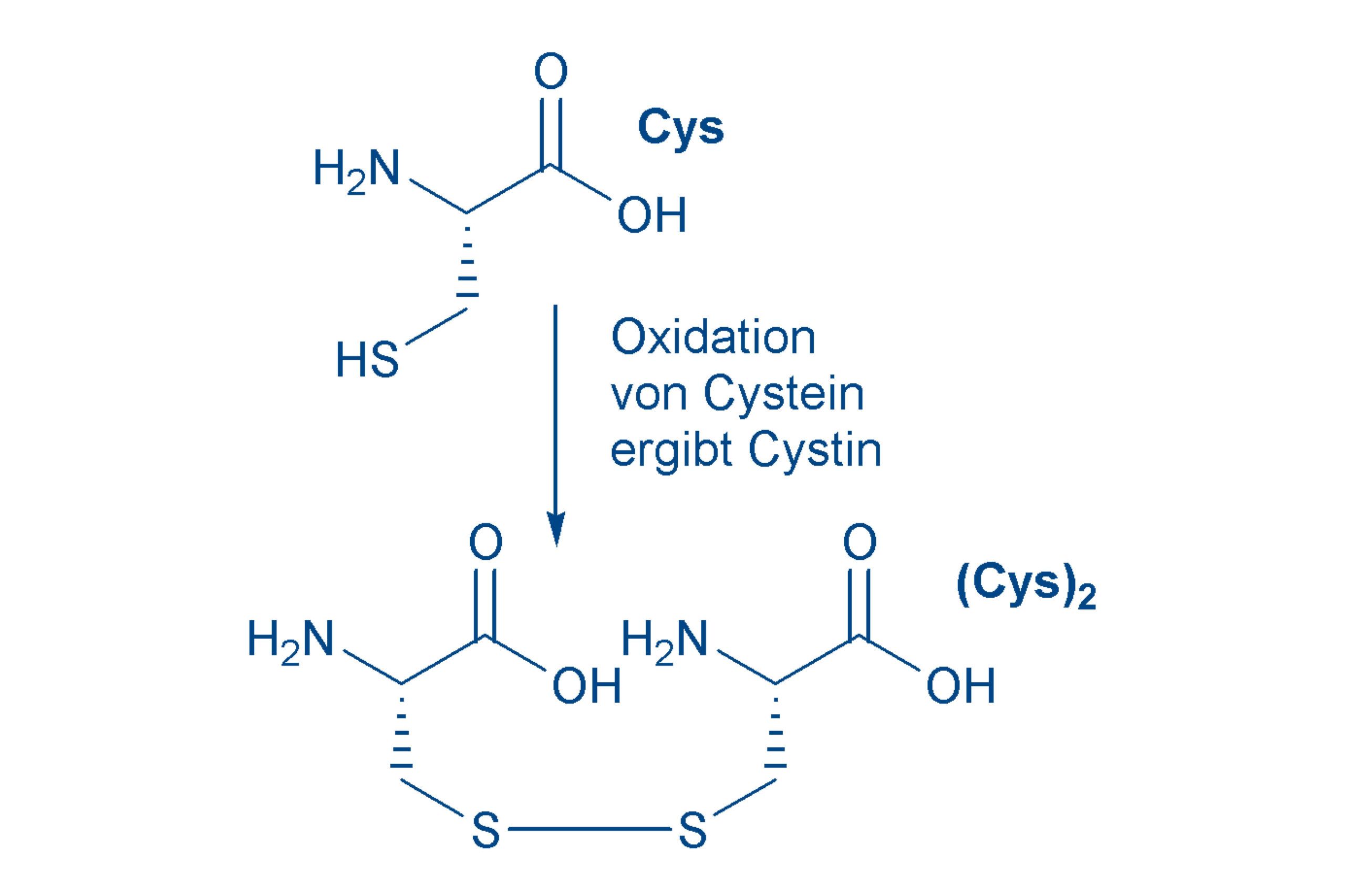
Cysteine (Cys, C)
is a rare amino acid, although it is extremely important for the structure of peptides and proteins. The non-essential Cys contains sulfur in the side chain, as a slightly acidic thiol group. Thiol groups are readily oxidized, with the formation of a disulfide bond. One molecule of cystine (Cyt or (Cys)2) is formed from two molecules of cysteine. In peptides that contain a Cys, two chains are linked together. If there are two Cys in the peptide, then a ring can be produced, as we will see again later.
Glutamic acid (Glu, E)
is an acidic amino acid. Glu is non-essential. The salts of glutamic acid are called glutamates and the sodium salt (monosodium glutamate, MSG) is known as a taste-enhancer. Glu has great physiological importance in our nervous system as a neurotransmitter. Glu forms pyroglutamic acid much less readily than glutamine.
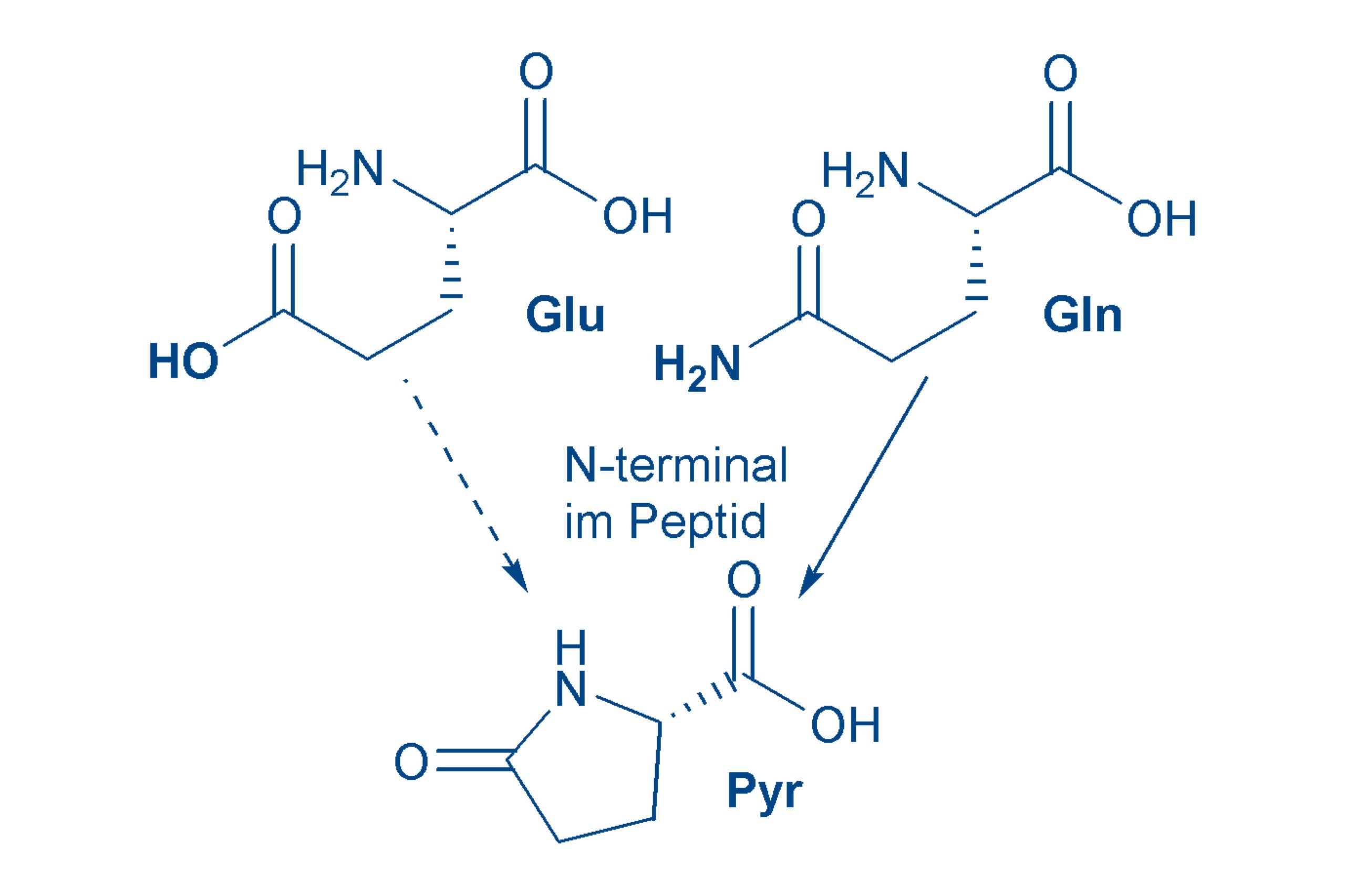
Glutamine (Gln, Q)
is actually a derivative (the amide) of glutamic acid. Glutamine is a polar amino acid that occurs as the free form in large quantities in the human body. Glutamine at the start of a peptide chain forms pyroglutamic acid spontaneously, or with the help of an enzyme.
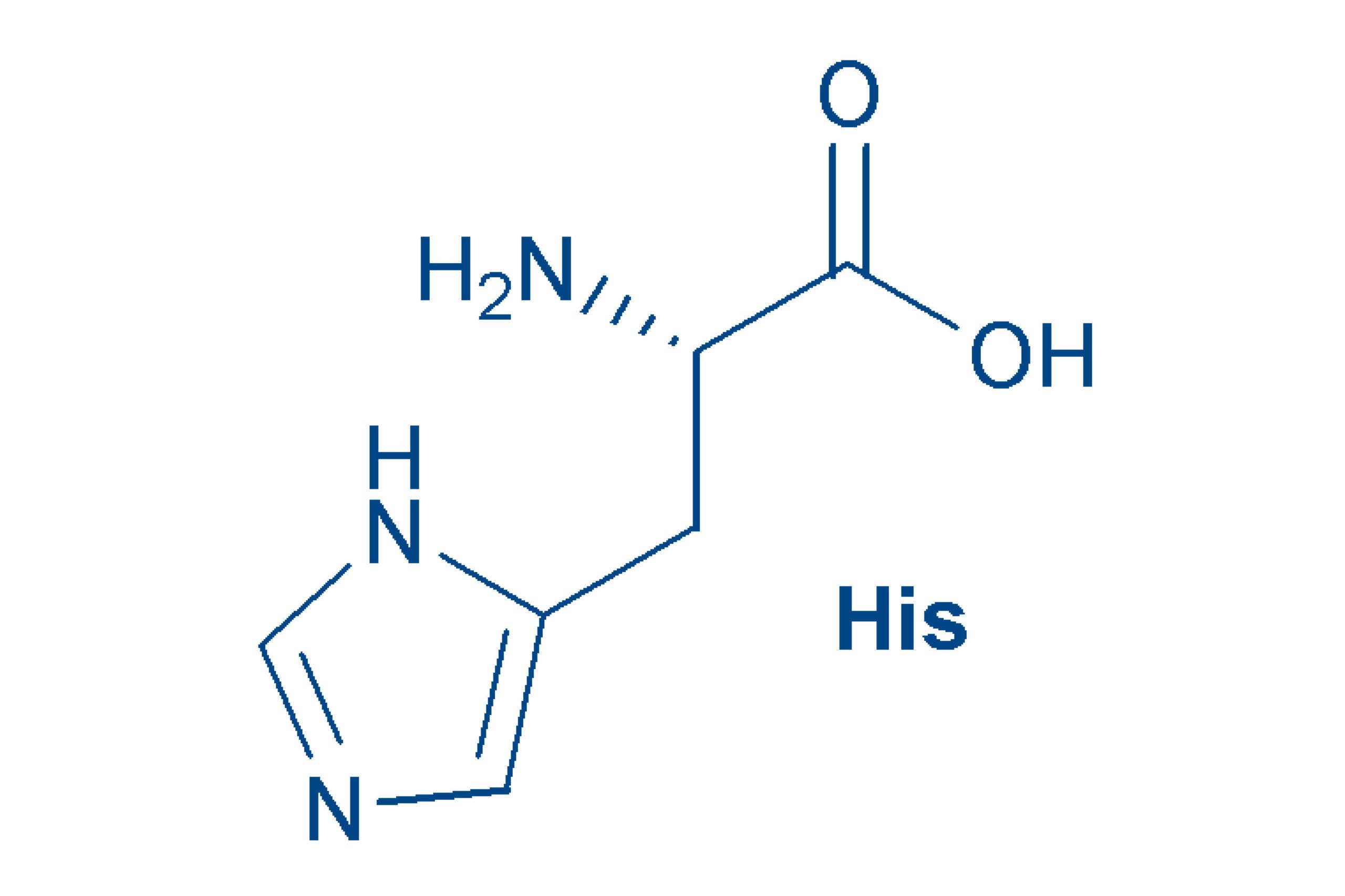
Histidine (His, H)
is a weakly basic, polar amino acid that is essential for humans. The name comes from “histos” (Greek for tissue). The imidazole ring that it carries in the side chain, contains two nitrogen atoms. Imidazoles catalyze many reactions and therefore His (in combination with Cys, Ser or Thr) is often to be found in the active center of enzymes.
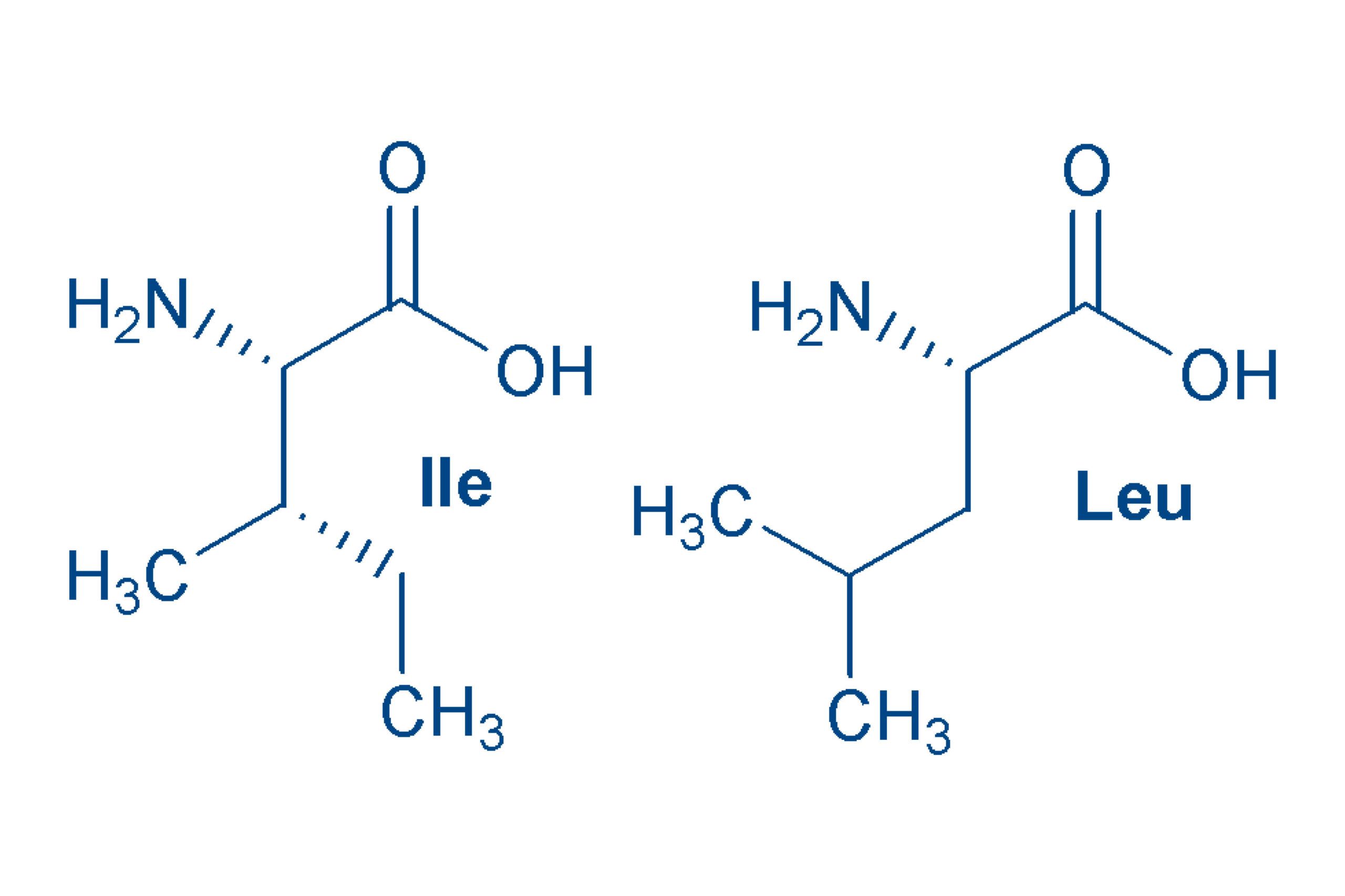
Leucine (Leu, L) and Isoleucine (Ile, I)
are isomeric α-amino acids. Isomeric compounds have the same molecular formula and the same molecular weight, but differ in their structure. Both are non-polar molecules. Leu and Ile are essential for humans. The name leucine comes from the white (Greek: leucos) leaves in which it crystallizes. Further stereoisomers are possible with isoleucine (allo-Ile).
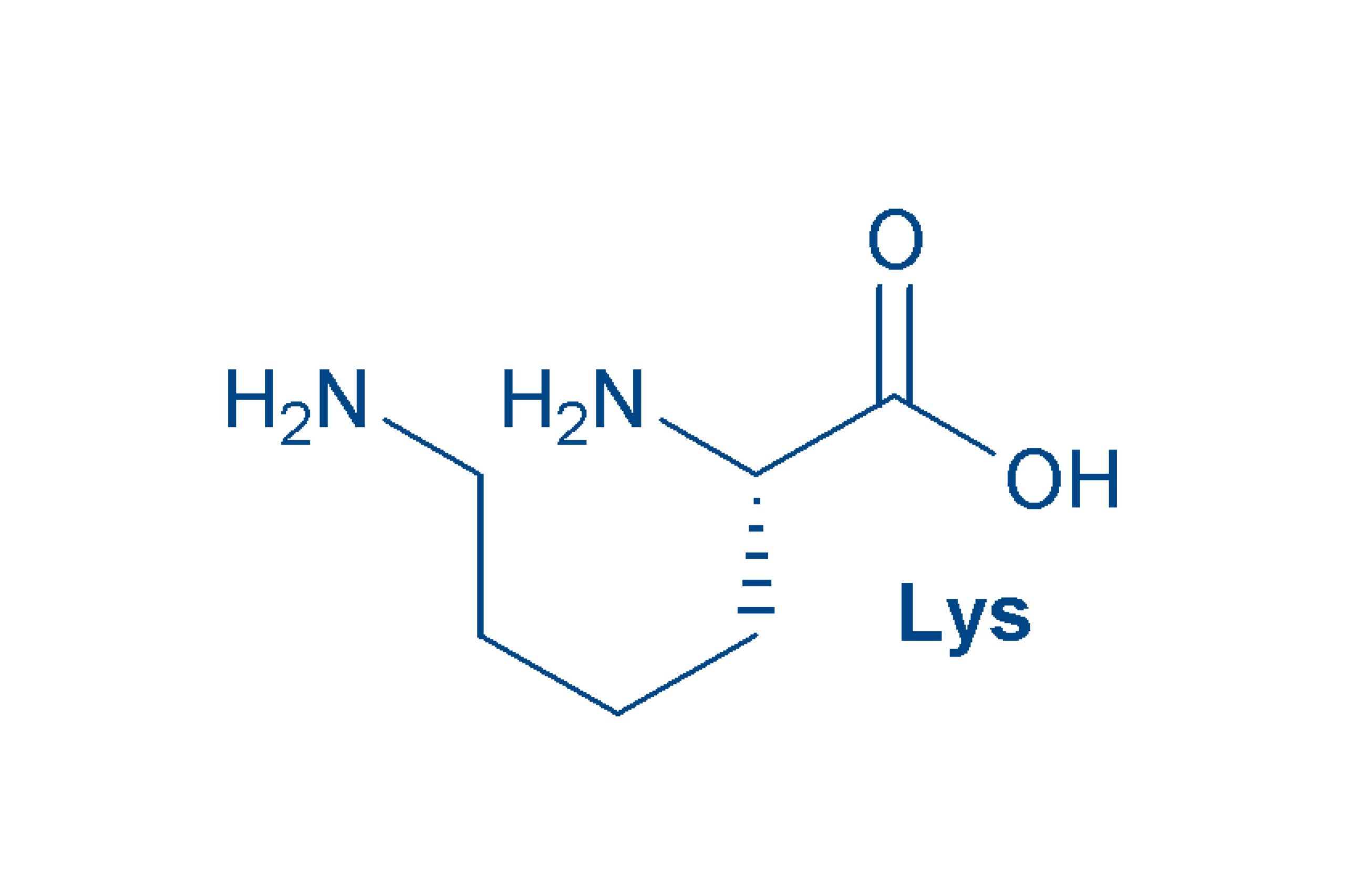
Lysine (Lys, K)
is a basic amino acid, that carries a second amino group in its side chain. It is an α,ε-
diamino acid. Lysine is essential.
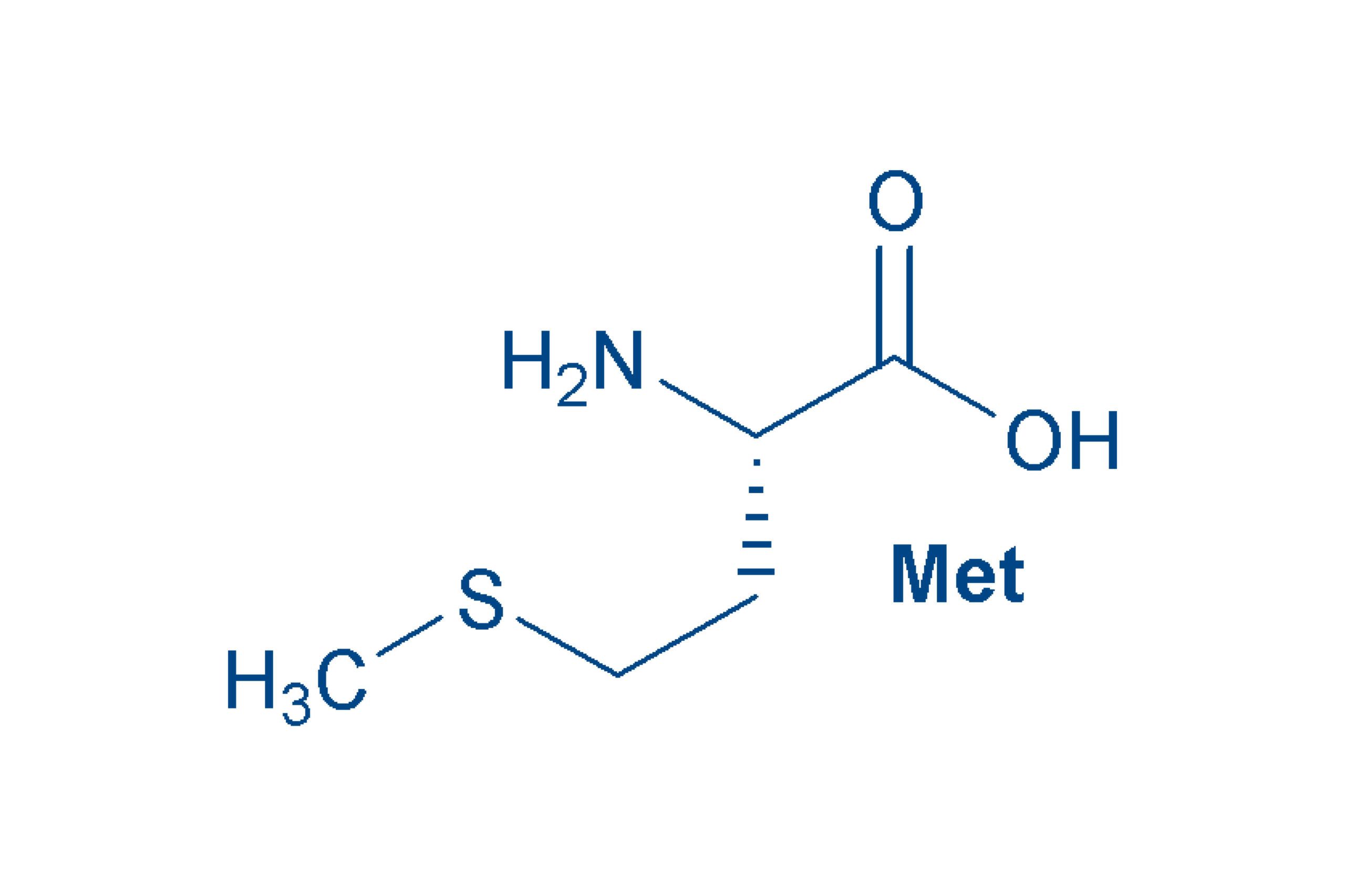
Methionine (Met, M)
is, like cysteine, a sulfur-containing amino acid. The sulfur is present as methyl thioether, as reflected in the name “Me-thio”. Thioethers are sensitive to oxidation, which should be heeded when handling Met-containing peptides. This essential amino acid is one of the non-polar amino acids.
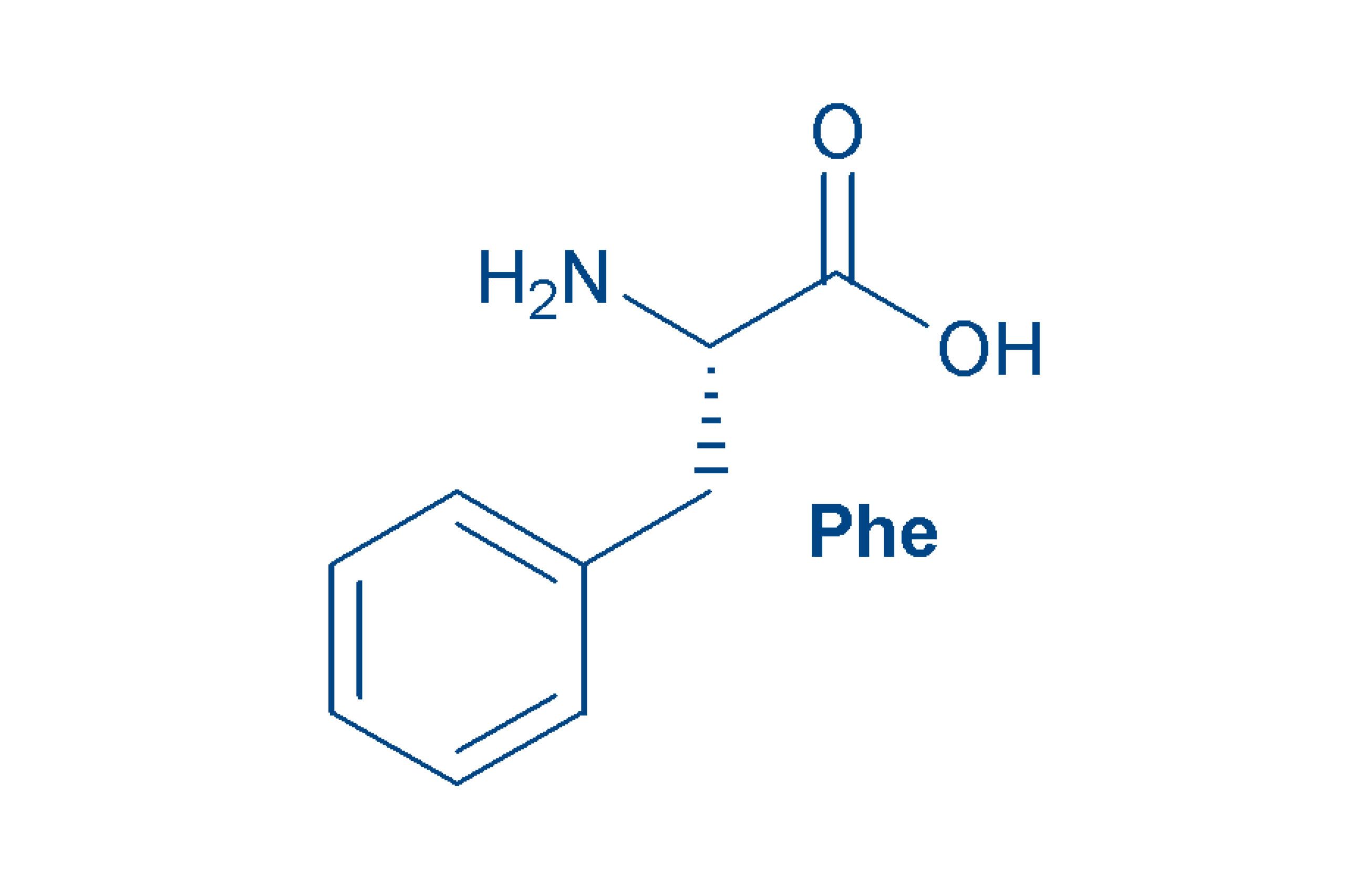
Phenylalanine (Phe, F)
is an essential, non-polar amino acid. Together with His, Tyr (that can be considered as a phenylalanine derivate) and Trp, it is one of the aromatic amino acids.
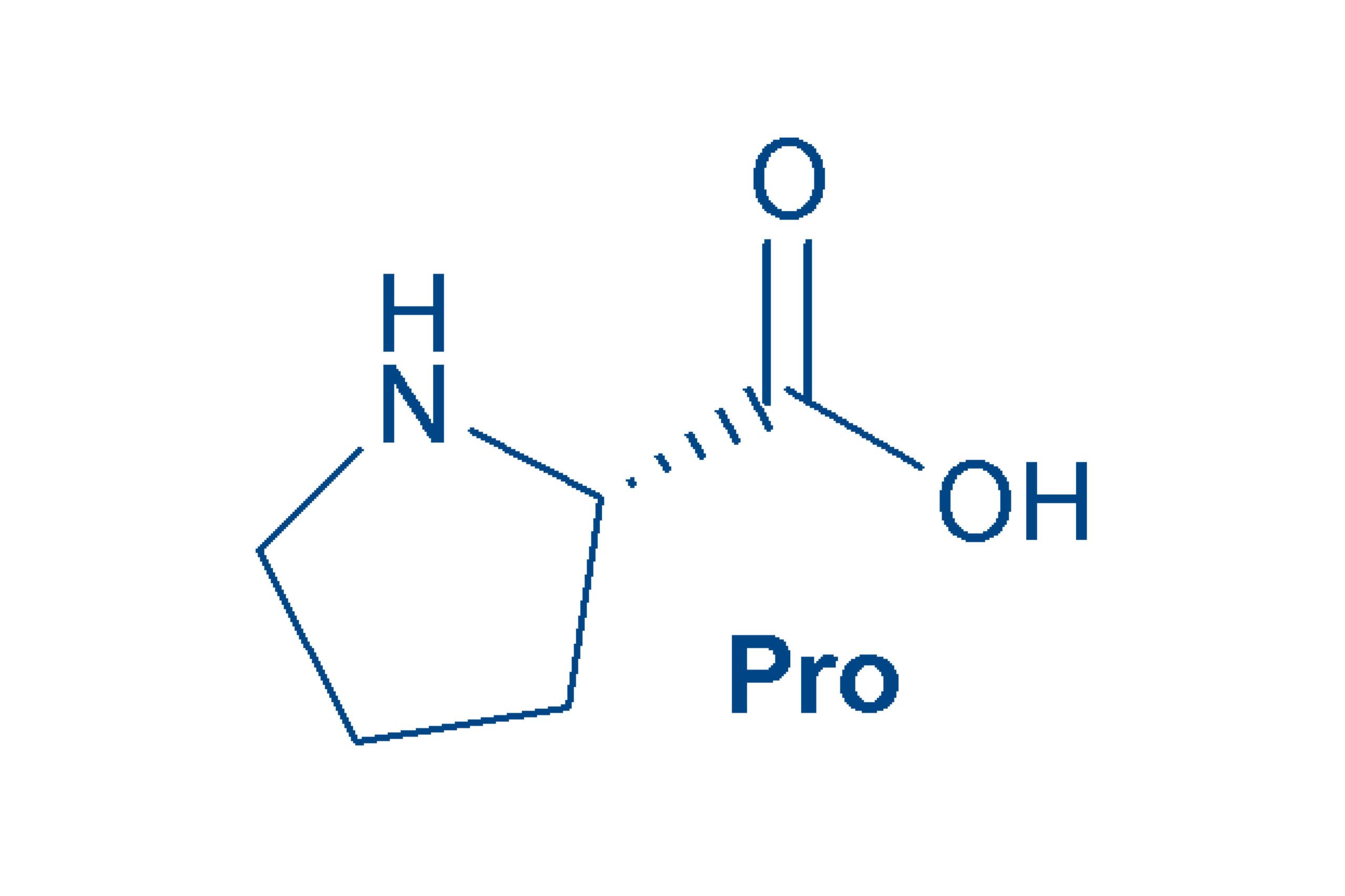
Proline (Pro, P)
is the only cyclic proteinogenic amino acid, a ring of 5 atoms that contains an α-amino acid group and an α-C. Pro is non-polar and non-essential. Due to its special structure, Pro has a huge influence on the spatial structure of peptides and proteins.
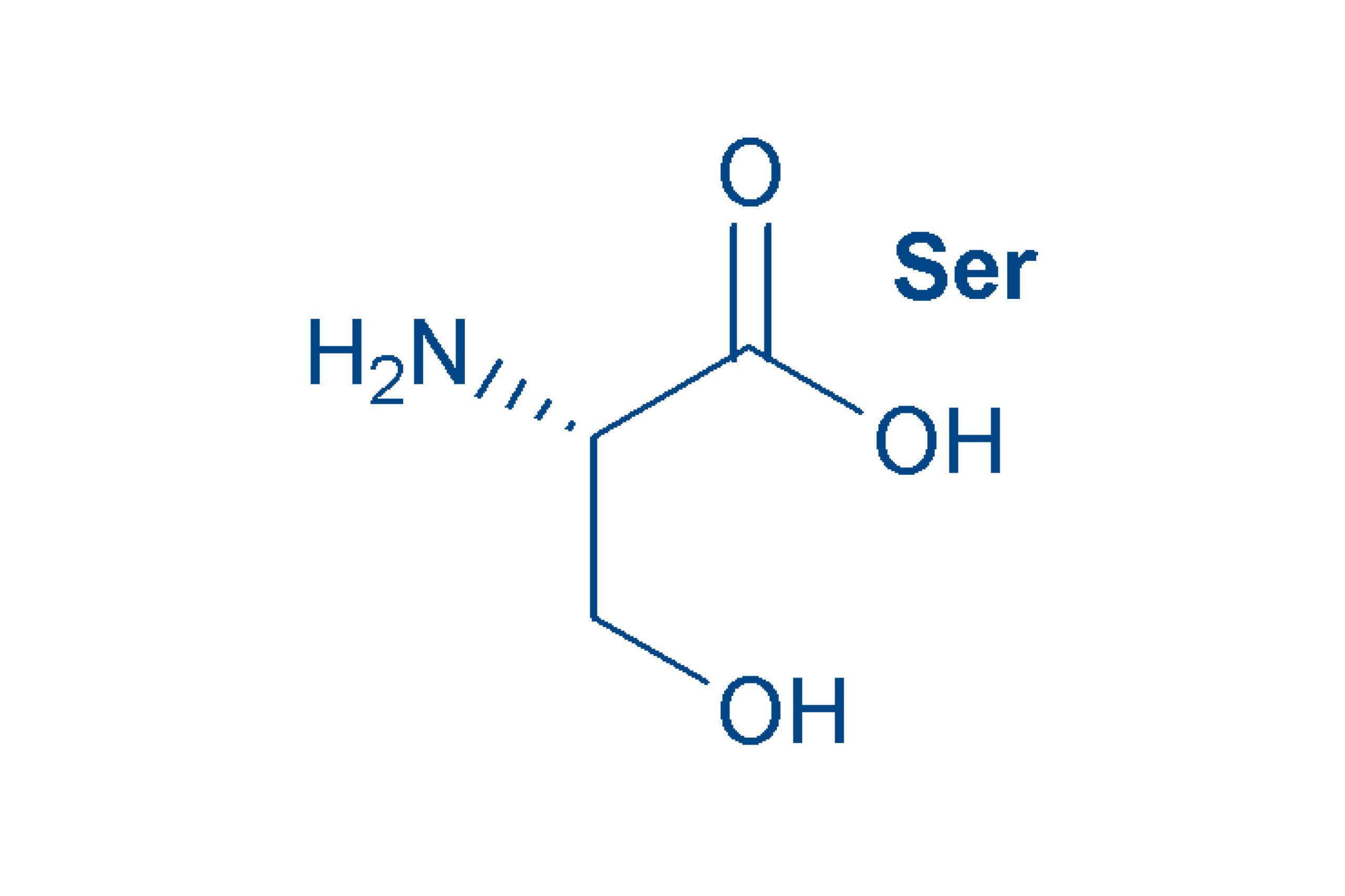
Serine (Ser, S)
is a polar amino acid due to the hydroxyl group in the side chain. The name of this non-essential amino acid is derived from the Latin (sericum) for silk.
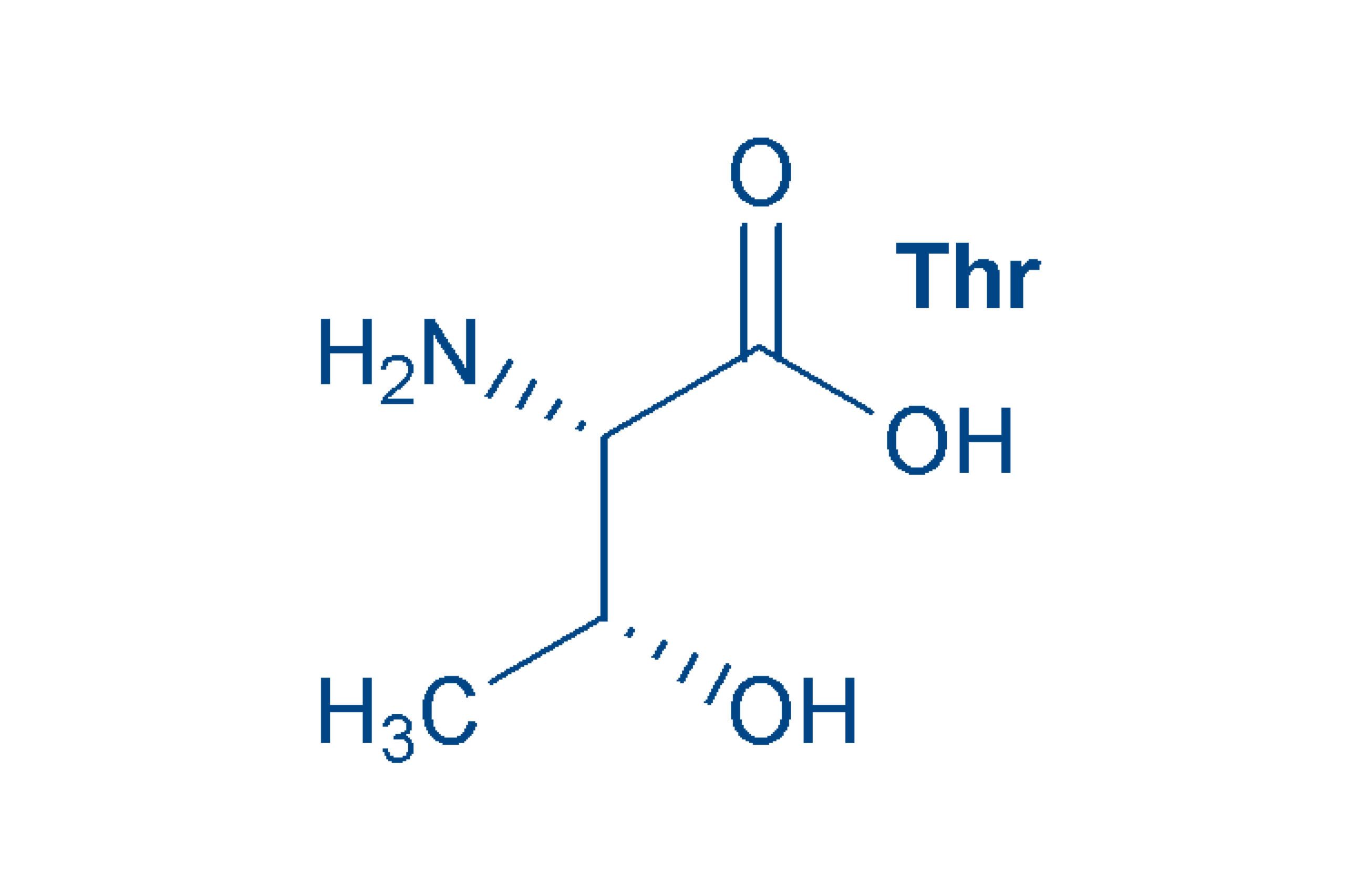
Threonine (Thr, T)
like serine, contains a hydroxyl group in the side chain, but is an essential amino acid. Threonine, in common with isoleucine, contains a second asymmetric carbon atom with four different substituents, so other stereoisomers are possible (allo-Thr).
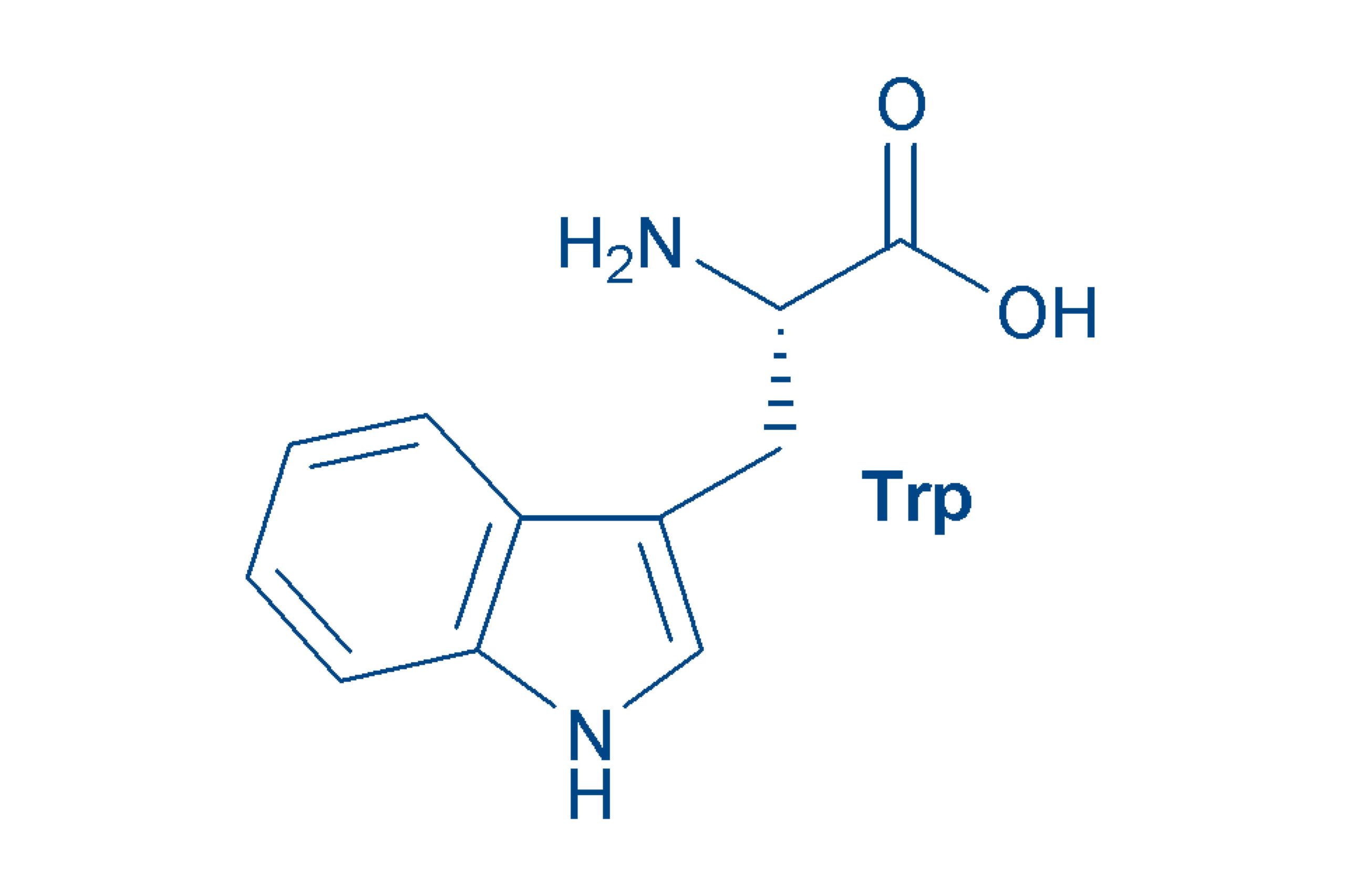
Tryptophan (Trp, W)
is a non-polar essential amino acid, an indole derivative. The free amino acid has an antidepressant effect and is a precursor of serotonin. Tryptophan fluoresces in the UV range (308-350 nm).
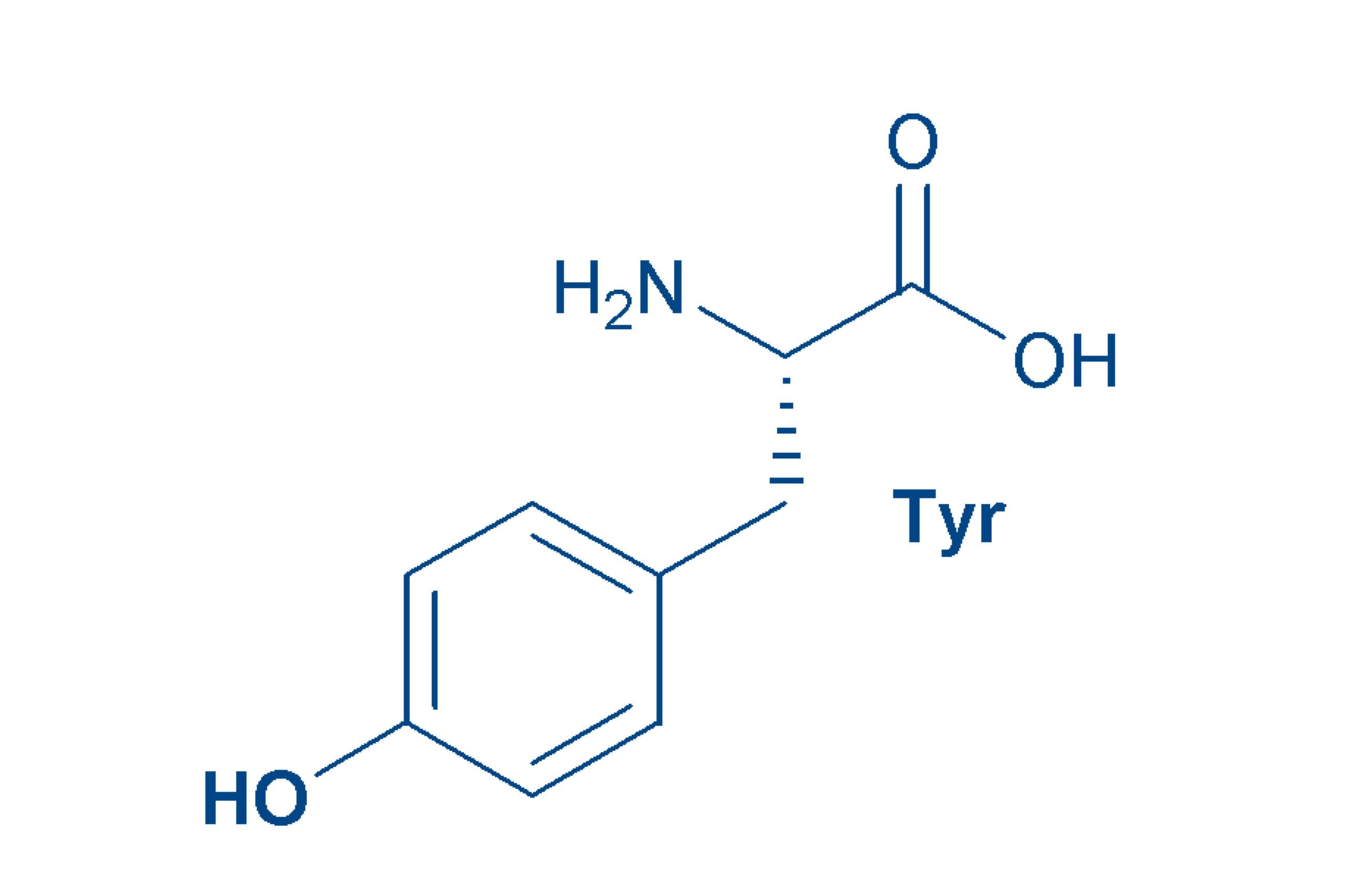
Tyrosine (Tyr, Y)
since it can be formed in the body from phenylalanine, tyrosine is a non-essential amino acid. The relatively non-polar amino acid was first isolated from cheese (Greek: tyros).
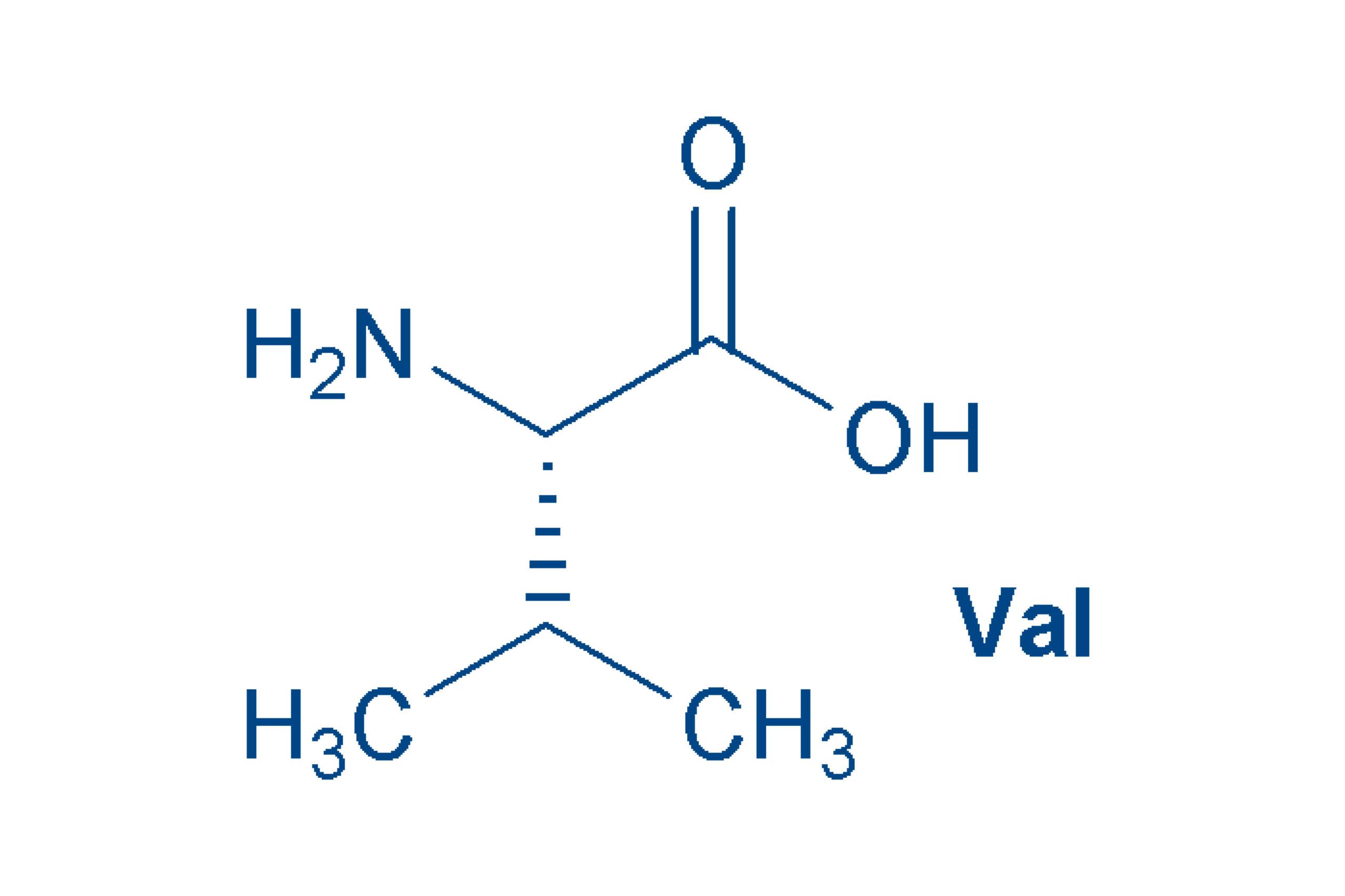
Valine (Val, V)
is a non-polar essential amino acid.
Read the article about what amino acids are
Click here >>
Watch the video about what amino acids are
Click here >>
Subscribe to our newsletter
"*" indicates required fields