Introduction
Bachem offers a number of N-protected amino acids as DCHA (dicyclohexylammonium) salts. The production of such salts is preferred if the free amino acid is not available in a crystalline form or if the free acid is unstable. In addition, in some cases DCHA salts of derivatives can be purified in a straight-forward process. An example represents the separation of racemates using their diastereomeric salts. However, before starting peptide synthesis, the amino acids have first to be liberated from their DCHA salts.
A structural procedure is outlined below:
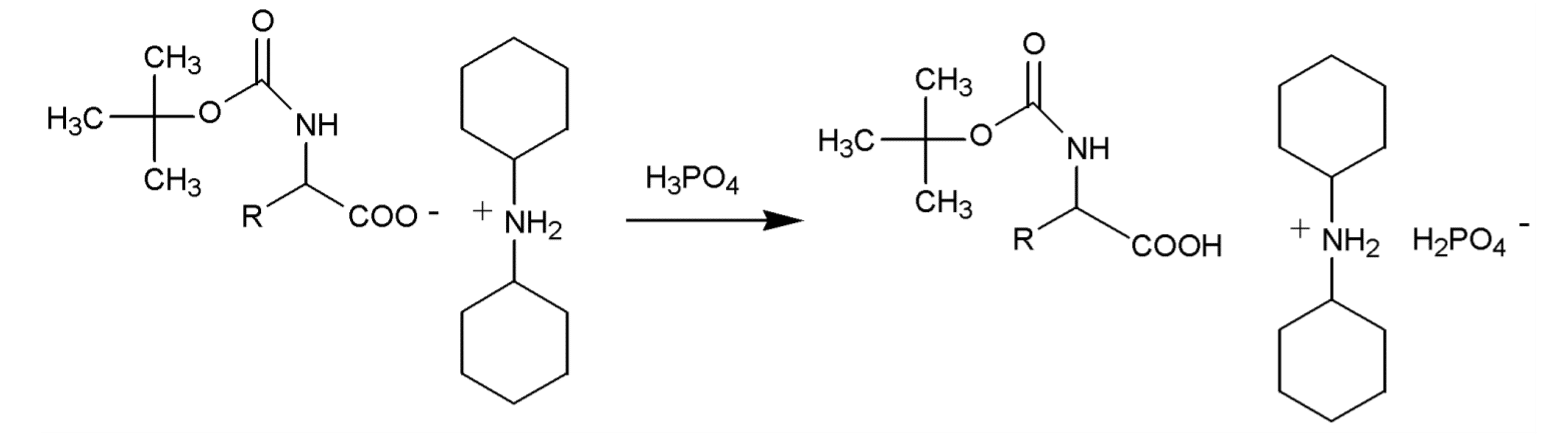
Typical procedure for the release of amino acids from their DCHA salts
- Suspend 1 part of the DCHA salt in 5 – 10 volume parts of ethylacetate, t-butyl methyl ether, isopropyl ether or in a mixture of these solvents.
- Use cold solvents (-20°C) in the presence of acid-labile protecting groups.
- Add phosphoric acid 10 % under stirring until the DCHA salt is completely dissolved and two clear phases appear. The pH of the lower, aqueous phase should read 2-3.
- Separate the aqueous phase and wash the organic phase once with 2 volume parts of phosphoric acid 10 % and extract again the organic phase three times with 2 volume parts of water. The pH of the aqueous phase should be ≥4.
- Check by TLC if all of the amino acid has been liberated from its DCHA salt.
- Dry the organic phase over anhydrous sodium sulfate, filtrate, and evaporate the filtrate to dryness in vacuo to obtain the free amino acid (most likely as an oil).
Notice
With hydrochloric acid dicyclohexylamine forms the sparingly soluble dicyclohexylammonium chloride. Therefore, the release of the amino acid from its DCHA salt has to be carried out using phosphoric acid as mentioned above. Alternatively, sulfuric acid 1M.W. Pennington and B.M. Dunn
Peptide Synthesis Protocols Methods in Molecular Biology, Vol. 35, p. 191, Humana Press, Totowa, New Jersey (1994) or KHSO4 2 M. Bodanszky and A. Bodanszky
The Practice of Peptide Synthesis, 2nd edition, p. 207, Springer Verlag, Berlin (1994)3R. Spannenberg, P. Thamm, and E. Wünsch
Hoppe-Seyler’s Z. Physiol. Chem. 352, 655 (1971) can be applied.
For the same reason, sodium chloride solution (brine) may be added to the washing buffer only after DCHA has been completely removed.
We hope you are successful in working with our products. Please don’t hesitate to contact us. We are here to provide you with any product information needed.
Subscribe to our newsletter
"*" indicates required fields
References
- 1M.W. Pennington and B.M. Dunn
Peptide Synthesis Protocols Methods in Molecular Biology, Vol. 35, p. 191, Humana Press, Totowa, New Jersey (1994) - 2M. Bodanszky and A. Bodanszky
The Practice of Peptide Synthesis, 2nd edition, p. 207, Springer Verlag, Berlin (1994) - 3R. Spannenberg, P. Thamm, and E. Wünsch
Hoppe-Seyler’s Z. Physiol. Chem. 352, 655 (1971)