The innovative PEC platform enables the efficient putification even of complex peptides
Overview
Innovation is a central pillar of Bachem’s success. Our dedicated teams explore and develop new technologies to bring innovative solutions to our partner’s needs. Here, we will introduce a new innovation that represents a new turn in peptide purification.
Nowadays, SPPS (solid phase peptide synthesis) is the go-to method for peptide synthesis. It is a rapid, orthogonal and efficient process. SPPS is able to synthesize sets of peptides in parallel, while the go-to purification method, RP-HPLC (LC), is a linear process and can represent the bottleneck of peptide development and manufacturing. The new purification method introduced in this whitepaper extends the previous features of SPPS to the purification of peptides. The catch-and-release platform, termed Peptide Easy Clean (PEC), developed by Belyntic in collaboration with Bachem, enables the successful and innovative purification of peptides. The open access Edge Article published in Chemical Science demonstrates the development of a new traceless linker for catch-and-release purification 1R. Zitterbart, N. Berger, O. Reimann, G. Noble, S. Lüdtke, D. Sarma and O. Seitz, Traceless parallel peptide purification by a first-in-class reductively cleavable linker system featuring a safety-release, Chem. Science (manuscript accepted). The linker presented improves upon the previous presented2O. Reimann, O, Seitz, D. Sarma and R. Zitterbart. A traceless catch-and-release method for rapid peptide purification. J Pep Sci. 2019; 25:e3136.3O. Reimann, D. Sarma, G. Noble, R. Zitterbart and O. Seitz, Enabling parallel Peptide purification by a novel traceless purification linker. 35th European Peptide Symposium (EPS), Conference paper, 2018. White papers published on Belyntic website in 2019), offering higher yield and fewer side-reactions.
- Increasing the speed of peptide development: PEC is universally applicable. It allows parallel purifications, in contrast to RP-HPLC (LC) which is a linear process.4O. Reimann, R. Zitterbart, G. Noble, Increased yield – PEC vs. Flash
- Facilitated purification of difficult peptides: PEC is an orthogonal approach to LC, it specifically removes capped peptide truncations 5O. Reimann, R. Zitterbart, G. Noble, The new dimension of
purity – Orthogonality. In addition, the peptide catch step is tolerant of a wide range of conditions, enabling loading in organic solvents that are typically incompatible with traditional LC. Furthermore, the linker can facilitate solubilization of aggregation-prone peptides through disruption of secondary structure. - Improving drug stability and efficacy: PEC is a modification platform for peptides. The innovative safety-release enables conjugation chemistries to explore the impossible through highly efficient solid-phase peptide modification.
This new platform could improve API lead generation for potential scale-up projects targeting difficult and complex peptides.
The PEC Platform
Peptides represent a great opportunity for therapeutic modalities, but pose significant challenges as well. One approach to enhance the pharmacokinetic and pharmacodynamic properties of peptides is direct chemical modification. The trend to longer and chemically modified peptides tends to make their synthesis and purification difficult. Long peptides or strongly hydrophobic peptides may aggregate and become challenging to handle, either for further chemical reactions or for purification, since they can self-assemble in certain solvents. The PEC platform represents an opportunity to overcome these drawbacks and may facilitate and/or accelerate the purification process. This technology allows peptide solubilization in organic solvents, enabling easier handling, and opens new opportunities to perform further chemical modifications. Furthermore, the invention provides an efficient and rapid purification that can be run in parallel for peptide libraries.
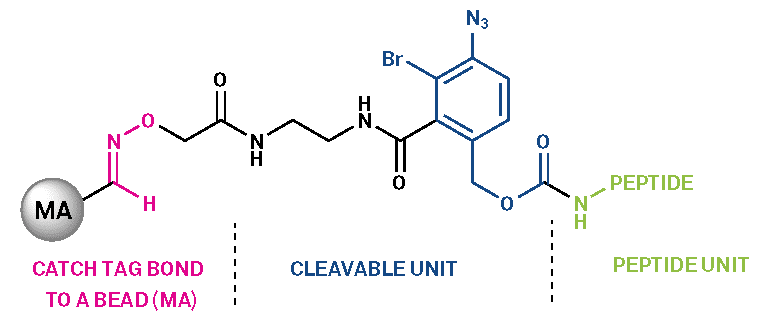
Figure 1 – Structure of the PEC purification linker (MA: modified agarose beads)
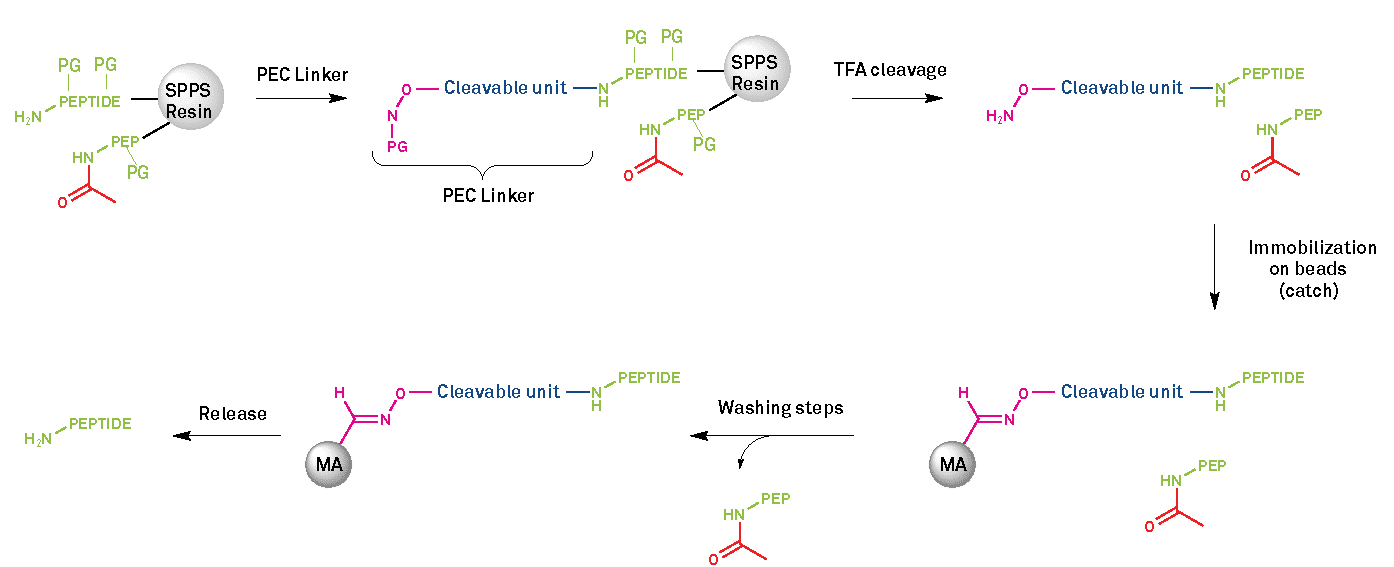
Scheme 1 – PEC platform mechanism (PG: protecting group; PEP: truncated sequences due to incomplete amino acids coupling)
The PEC Process
Linker Attachment
Prior to the purification steps, the target peptide is synthesized through the usual SPPS process, following regular cycles of deprotection and amino acid coupling. During the synthesis, the capping steps with acetic anhydride are a key factor. Capping is required in order to deactivate any unreacted site on the growing chain of the peptide, by leaving an acetyl group (Scheme 1, red color) on the N-terminus of unreacted sequences. Thus, they cannot participate in the next amino acid coupling cycles, preventing the formation of deletion sequences caused by difficult couplings.
The first step of this new method starts after completion of synthesis by the coupling of the PEC linker on to the N-terminus (or other free amine) of the full-length peptide. The PEC linker is covalently bound to the peptide as a carbamate. This coupling is straightforward and can be automated by the use of a peptide synthesizer.
Cleavage and Catch Steps
Once the linker has been successfully added, the second step of this process is TFA cleavage of the peptide off the SPPS resin with concomitant removal of side-chain protecting groups and PEC linker unmasking for the next step, the catch. The careful use of cleavage scavengers is essential to prevent the re-addition of the protecting groups on the peptide, thus altering the final peptide. At that stage, the presence of the linker on the full-length peptide can improve the solubility of hydrophobic and aggregation-prone peptides in organic solvents, such as DMSO (dimethyl sulfoxide) or HFIP (hexafluoro2-propanol). This can aid handling of the crude peptide by preventing self-assembly and formation of aggregates. This feature affords the PEC linker a second application, a modification platform. The third step in the PEC process is the catch. The linker-modified peptide is attached to agarose beads via an oxime ligation. This step can be performed in a variety of organic solvents that are typically incompatible with traditional LC methods. Then, with repetitive washing steps, all unmodified and truncated sequences, as well as chemical impurities, are removed. At this stage, the unprotected peptide bound to the purification platform is ready to undergo solidphase modifications, circumventing difficulties that are encountered for modification in the solution phase, such as solubility issues.
Release
The final step is the traceless release of the purified full-length peptide from the linker via reductive (dithiothreitol (DTT)) solution, followed by weak acid hydrolysis. Prior to hydrolysis, chemical modification can be performed on the unprotected peptide, such as lipidation, disulfide formation and other click-type reactions.
The final acidic treatment safely liberates the peptide by an acidcatalyzed1,6-elimination without causing any side-reactions. This safety release under mild conditions ensures peptides can be liberated while retaining chemical modifications performed during the catch step. Finally, the pure peptide is recovered after washing steps and optional ether precipitation.
Rapid Development
PEC has been applied for the purification of a set of 20 peptides of a personalized peptide vaccine; purification was performed in parallel and completed within 6 hours. In contrast, traditional single batch processing via RP-HPLC could take several days to achieve a similar result. This example demonstrates the great potential of this technology for the rapid development of peptides, additionally to provide a convenient, efficient modification and general purification platform.
Limitations
The PEC process is capable of removing capped impurities from incomplete coupling only, therefore use of capping after every amino acid coupling step is essential. Other impurities routinely formed during SPPS, such as point deletions caused by incomplete Fmoc-deprotection, racemization during activation, aspartimide formation, etc, cannot be removed by PEC, therefore limiting the maximum purity achievable, necessitating a further purification step to reach maximum purity. However, a combination of PEC and traditional LC can be utilised effectively to maximise final product purity as demonstrated for Histone H3 (1-20) [5].
Conclusion
To conclude, the PEC platform represents a new innovation to tackle the efficient and successful development and production of complex peptides, such as long peptides, hydrophobic peptides and modified peptides. The traceless safe release of the peptide from the PEC platform represents a great opportunity to explore more challenging modifications without compromising peptide structure. The collaboration demonstrates Bachem’s dedication to providing solutions to difficult problems through innovation.
With the expertise of Bachem in the development and manufacturing of peptide APIs, together with our industry partner Belyntic, we once more underline our position as Leading Partner in Tides.

Dr. Gavin Noble
Dr Gavin T. Noble is the Research and Development Chemist at Bachem UK St Helens and had the leading role at Bachem in the development of the PEC platform. At BUK his primary focus is undertaking and coordinating investigations into new production methods for research-grade peptide manufacture, as well as supporting the Synthesis and Purification groups in a troubleshooting capacity. He has been employed at Bachem since 2014 and spent 3 months at Bachem Bubendorf in 2015 as part of a know-how exchange program, working in R&D I as a Project Chemist. Prior to his position at Bachem, he worked as a post-doctoral fellow at The University of Notre Dame (USA), researching peptide-targeted liposome nanoparticles for cancer and antimalarial therapies. He obtained his PhD from The University of Manchester (UK), performing research into protein and enzyme activity at synthetic liposome surfaces. During his career, he has published several peer-reviewed articles in highimpact journals, with over 400 citations.
About Belyntic
The Belyntic GmbH is a Chemistry-for-Healthcare company. Belyntic offers solutions for the development and production of peptide-based drugs using their proprietary Peptide Easy Clean (PEC) technology. The company’s headquarters are located at the Adlershof science and technology park, Richard-illstätter-Str. 11, 12489 Berlin, Germany. For further information, please visit the Belyntic website or contact Dominik Sarma, co-managing director, at 030 8104-1113 or by email via dominik.sarma@belyntic.com.
Subscribe to our newsletter
"*" indicates required fields
References
- 1R. Zitterbart, N. Berger, O. Reimann, G. Noble, S. Lüdtke, D. Sarma and O. Seitz, Traceless parallel peptide purification by a first-in-class reductively cleavable linker system featuring a safety-release, Chem. Science (manuscript accepted)
- 2O. Reimann, O, Seitz, D. Sarma and R. Zitterbart. A traceless catch-and-release method for rapid peptide purification. J Pep Sci. 2019; 25:e3136.
- 3O. Reimann, D. Sarma, G. Noble, R. Zitterbart and O. Seitz, Enabling parallel Peptide purification by a novel traceless purification linker. 35th European Peptide Symposium (EPS), Conference paper, 2018. White papers published on Belyntic website in 2019)
- 4O. Reimann, R. Zitterbart, G. Noble, Increased yield – PEC vs. Flash
- 5O. Reimann, R. Zitterbart, G. Noble, The new dimension of
purity – Orthogonality