A valuable tool for peptide and protein modification
Bachem highlights the importance of «click reactions» in peptide chemistry as a simple and versatile concept for peptide synthesis and chemoselective modification. The broad spectrum of applications of the reaction includes ligation, cyclization, bio-conjugation, and radiolabeling of peptides.
“Click Chemistry” is a term introduced by K.B. Sharpless, H.C. Kolb, and V. V. Fokin from the Scripps Research Institute at La Jolla to describe chemistry tailored to generate substances quickly and reliably by joining small units together similar to the modular strategy adopted by Nature. The term “click chemistry” applies to reactions that are highly efficient, wide in scope, and stereospecific. Product isolation is easy, the reactions are simple to perform using inexpensive reagents and can be conducted in benign solvents such as water. The Huisgen 1,3-dipolar cycloaddition is probably the most extensively studied click reaction. A variant of this reaction, the copper-catalyzed azide-alkyne cycloaddition (CuAAC) independently developed by the groups of Sharpless at Scripps and Morten Meldal at Carlsberg Laboratory in Denmark fits the «click chemistry» concept well and is one of the most popular prototype click reactions to date.
Principle of click chemistry
A click reaction must be modular, wide in scope, high yielding, create only inoffensive by-products (that can be removed without chromatography), be stereospecific, simple to perform and require benign or easily removable solvents.
Prof. K. Barry Sharpless
(Nobel laureate in 2001)
Angew. Chem. Int. Ed. 40, 2004 (2001)
Chemistry of CuAAC
The popularity of the CuAAC is largely a result of the unique properties of both azides and the resulting triazoles. CuAAC involves the formation of a 1,2,3-triazole ring which is a rigid five-membered heterocycle. Such triazoles are isosteres of the peptide bond, mimicking the planarity of the amide moiety, but less prone to hydrolytic cleavage (Figure 1).
Most click reactions involve carbon-hetero atom bonding processes and have a high energy content which make the additions irreversible. Furthermore, azide moieties are easy to introduce, stable to water and oxidative conditions, orthogonal to many commonly used functional groups and vigorously reactive with others. For applications in vitro and in vivo, azides are virtually absent from any naturally occurring species (bio-orthogonal). The combination of the robustness of the triazole bond, the resemblance to an amide bond, and the potential biological properties it could endow make the triazole linkage not merely a benign, easily synthesized linker, but an integral part of the success of click chemistry.
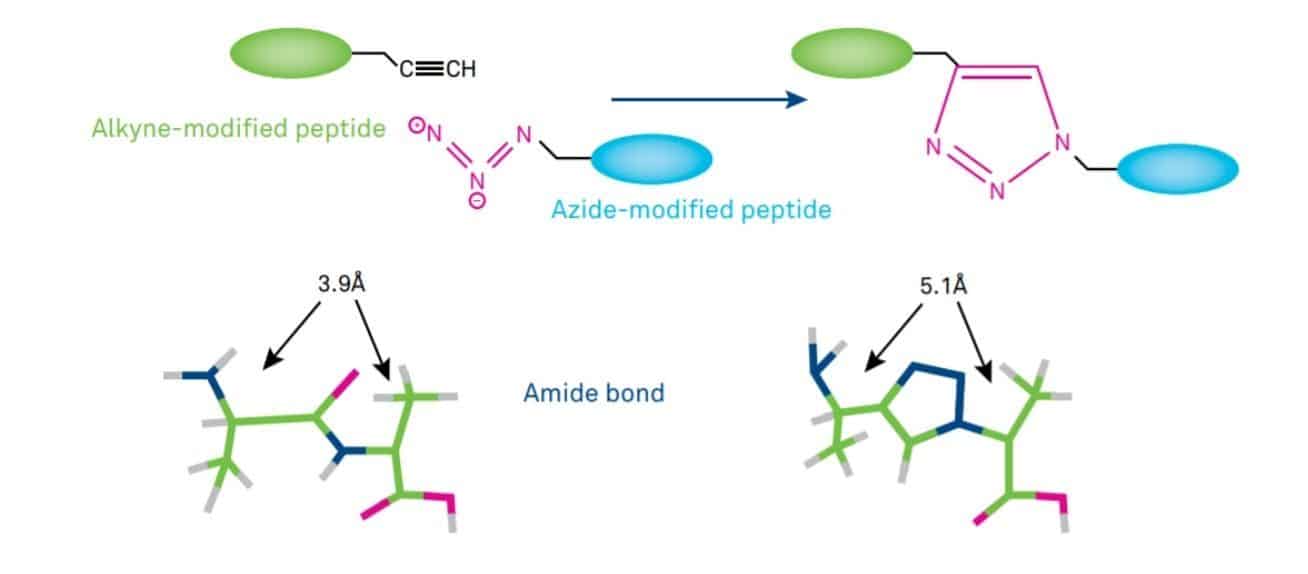
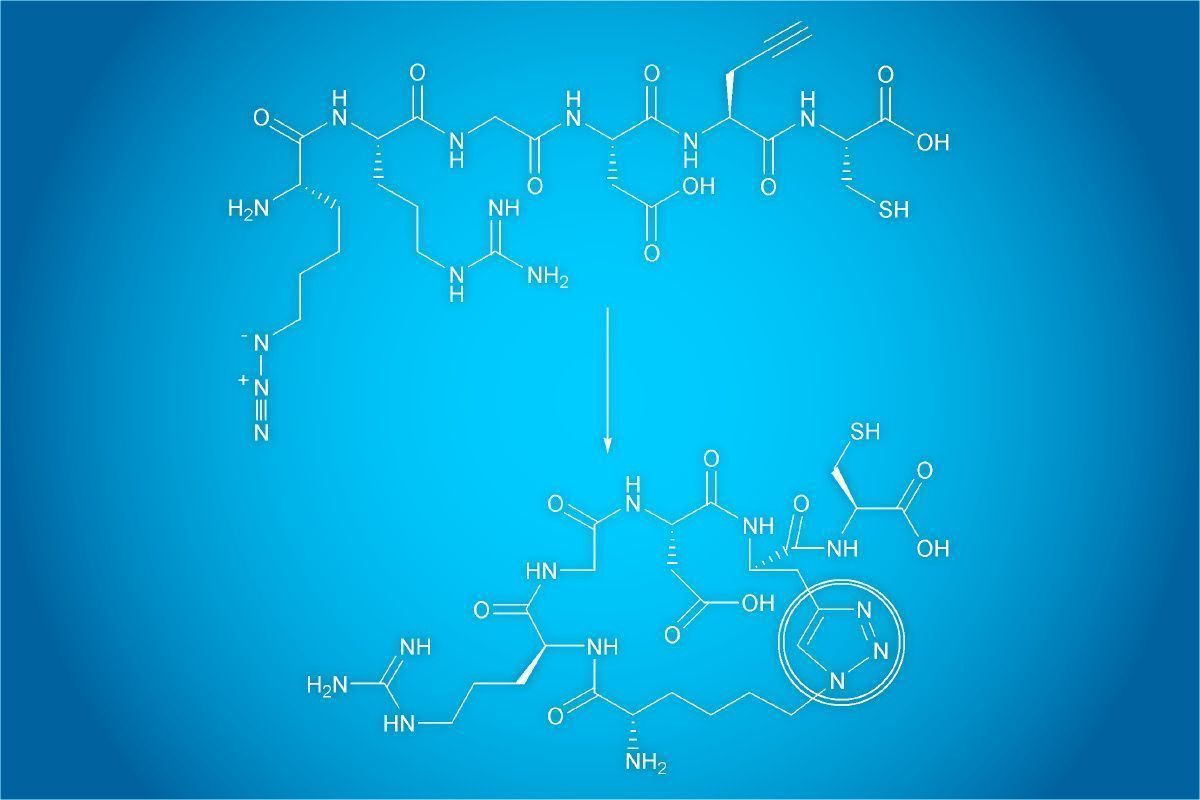
Figure1: Clicking of an alkyne-functionalized peptide with an azide-modified substrate in the presence of Cu(I) to form a triazole-linked conjugate.
Triazole linkage resembles an amide bond due to their relative planarity and strong dipole moments.
Click chemistry involving peptides
Click chemistry provides a number of avenues for peptide/protein modifications and could be combined with other techniques to make complex structures and multi-component functionalized systems with ease. The chemistry could be performed in different ways. For example, peptides can be converted post-synthetically to an azido derivative which can be clicked with appropriate substrate containing a clickable alkynyl group or vice versa. Peptides can also be made by inter- and intramolecular click reactions using azide or alkyne containing amino acids or building blocks during peptide synthesis. Building blocks containing clickable moieties will be instrumental for constructing sidechain modified peptides, interside-chain peptide chimera, peptide small molecule conjugates, and cyclic peptides. Solid phase resins modified with clickable groups can also be used for making clickable/modified peptides. Click chemistry is compatible with various protected amino acid side chains used in peptide synthesis.
A number of reagents and building blocks can be used for click chemistry. These include ω-azido-α-amino acids, PEG and spacer azides and alkynes, azide- or alkyne-modified fluorescent dyes and quenchers, nucleosides and nucleotides, alkyne or azide-containing chemical modification reagents, diazo transfer reagents, and propargyl derivatives of amino acids (e.g.O-propargylserine, glutamic acid bispropargyl amide).
The most important applications of click chemistry in peptide science include chemical ligation, cyclization and bio-conjugation (Figure 2). Other typical applications are conjugation of isotope labels for imaging, synthesis of peptidomimetics based on the triazole backbone, conformational and backbone modifications.
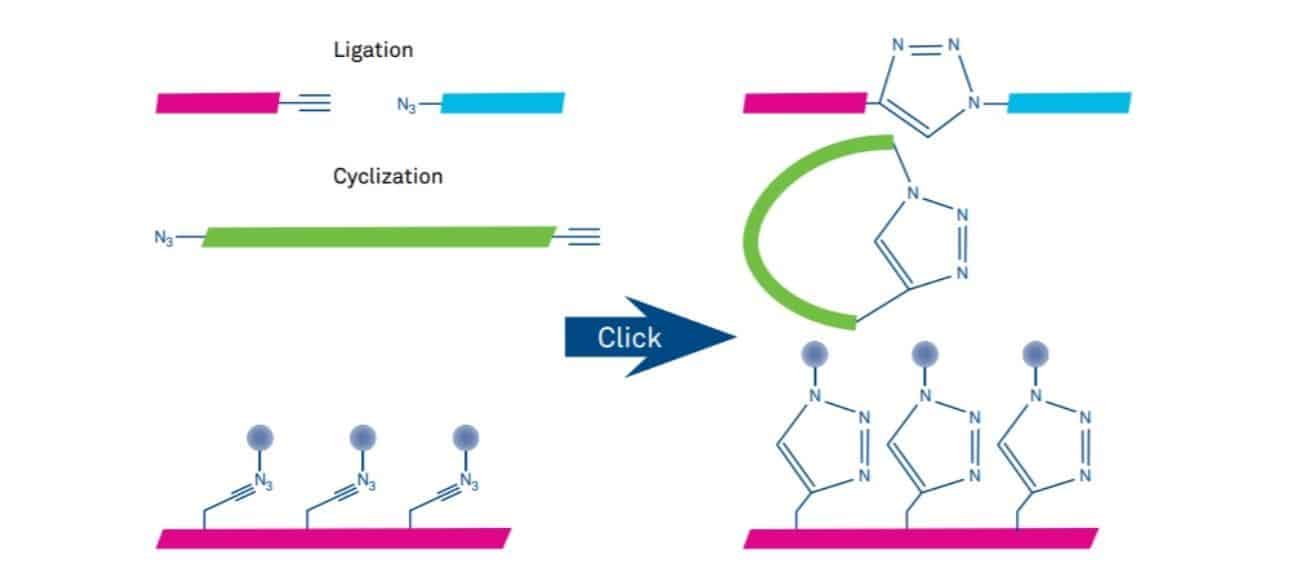
Figure 2: Utilization of click chemistry for ligation, cyclization and conjugation of peptides.
Chemical ligation and peptide modification
Linking two or more peptide fragments together to make a larger peptide chain is called ligation. Click chemistry can be conveniently utilized to make peptide–peptide linkages. A peptide fragment functionalized with an alkyne group could be ligated to another peptide with an N-terminal azide moiety resulting in a triazole linker (similar to an amide bond as explained earlier) holding two peptide units together.
Similarly multimeric peptides can be obtained by incorporating orthogonal side chain protecting groups such as ivDde or Aloc (for modifying the side chain of Lys) followed by selective deprotection, attachment of an alkyne function and clicking with N-terminal azide peptides. Numerous examples of peptide ligation have been published such as:
- synthesis of a clickable RGD peptide (obtained by reacting Lys side chain with azido acetic acid) that can be linked to another peptide fragment
- synthesis of a cell-permeable peptide therapeutic by clicking the alkynyl-modified peptide drug (using inexpensive propargylamine or 1-(2-nitrophenyl)propargyl alcohol) with nona-arginine modified with an azide group
- synthesis of neurotensin (8-13)-containing heterodimers by clicking alkyne-neurotensin (obtained by reacting with succinimidyl-hex-5-ynoate, the NHS ester of Bachem
product 4066756) with the azide of a Plk1-PBR binding phosphorylated hexapeptide. The resulting triazole-containing oligopeptides were found to self-dimerize in a headto-tail fashion as the native peptides.
Modification of peptides by PEGylation has been achieved by click chemistry. For example, a lipopeptide was assembled by solid-phase synthesis followed by an onresin PEGylation reaction (using azido-PEG) and cleavage of the PEGylated peptide from the resin. There is a tremendous potential for click chemistry for various chemical modifications of peptides and proteins (e.g. attaching ligands, liphophilic or hydrophilic groups or linkers etc.).
1. Peptide Cyclization:
A variety of macrocyclization methods are available to increase the clinical efficacy and bioavailability of peptides. The click reaction has been exploited in a number of different cyclization reactions such as the on-resin cyclization of a disulfide-containing peptide before or after removal of the side-chain protecting groups; the preparation of novel heterodetic cyclopeptides containing a triazole bridge by an intramolecular side chain-to-side chain click reaction; Cu(I)- and Ru(II)-mediated click cyclizations of tripeptides for generating vancomycininspired mimics; on-resin cyclization of peptide ligands of the vascular endothelial growth factor (VEGF) receptor-1 etc.
In many cases, formation of considerable amounts of macrocyclic heterodimers was observed during click-mediated macrocyclization reactions, opening up the prospects of synthesizing complex peptide structures, which are otherwise difficult to obtain. A novel stapling methodology for 3(10)-helical peptides using CuAAC click reaction in a model aminoisobutyric acid (Aib)-rich peptide resulted in a more ideal 3(10)-helix than its acyclic precursor.
2. Bioconjugation:
Bioconjugation is the process by which synthetic molecules are attached to biological targets, or by which biomolecules are linked together. The impact of click chemistry on bioconjugation has been extensive in recent years. Arginine-rich TAT peptides modified with a clickable azido group can be conjugated to oligonucleotides, cytotoxic drugs, kinase inhibitors etc. to facilitate cell penetration for therapeutic purposes. The application of the CuAAC reaction provides a powerful chemical method to access mimetics of glycopeptides and glycoproteins (neoglycopeptides and neoglycoproteins) of well-defined homogeneous structure. Complex cyclopeptide-centered multivalent glycoclusters has been synthesized using the Cu-catalyzed click reaction. Selfassembling peptide fibers can incorporate multiple clickable peptides non-covalently, stoichiometrically and without disrupting their structure or stability. They can be conjugated to biotin followed by streptavidin-nanogold particles, or rhodamine, and visualized by electron and light microscopy. This approach allows the development of multi-component functionalized systems. The click reaction allows conjugating fluorescent molecules to peptides and proteins under mild conditions, a most important application in the emerging field of cell biology and functional proteomics.
3. Peptidomimetics Design, Synthesis and Drug Discovery:
Due to its relative planarity and large dipole moment, the 1, 2, 3-triazole function formed by click reaction bears a physicochemical resemblance to an amide bond. Consequently, the triazole linkage has found particularly broad use in the field of peptidomimetics. The triazole unit is resistant to enzymatic degradation, hydrolysis, and oxidation, making it an attractive moiety to replace more labile linkers in biologically active compounds. The click reaction has been utilized as a conjugation strategy in the design and synthesis of complex biomimetic architectures in which the triazole linkage replaces, and in some cases acts as a surrogate for peptide and phosphodiester bonds. Replacing a peptide bond with a triazole unit could result in interesting structures with unique conformational characteristics. Triazole units formed by the click reaction can act as helical component, a β-turn unit and a cis/trans-prolyl ratio modifier. Triazole units can also act as an effective replacement for a peptide portion in HIV-1 protease inhibitors. Modified peptides in which a triazole ring is introduced in the peptide backbone or attached to the side chain of a residue are good candidates to design new antimicrobial agents.
4. Radiolabeling and Imaging:
The CuAAC is an ideal ligation reaction for radiolabeling sensitive biomolecules. Alkyne or azide derivatives of radioisotopecontaining compounds could be used for labeling biomolecules such as folic acid, peptides, proteins, and glycopeptides. For example, an 11C isotope label was introduced via converting [11C]-CH3 I into [11C]-CH3 N3 by nucleophilic substitution and subsequently reacting the azide with an alkyne-modified peptide. 18F labeling for PET imaging was achieved by clicking azidomethyl-4-[18F]-fluorobenzene to a modified peptide. An important limitation of CuAAC should not be left unmentioned: Chelators as DOTA will form a complex with the catalyst, so conjugates with such compounds are more difficult to obtain.
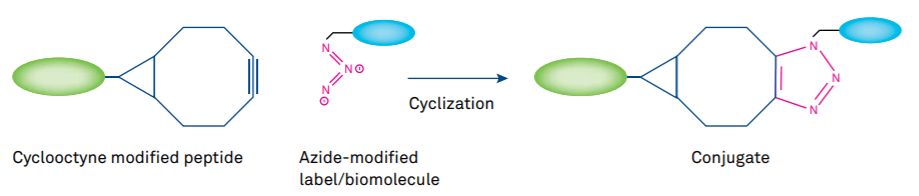
Figure 3: Copper-free click reaction using cyclooctyne-based substrates
Cu-free Click Reactions
Additionally, the cytotoxicity of copper remains a concern and a limiting factor for the widespread in vivo application of the CuAAC reaction.
Meanwhile, Cu-free alternatives have been developed. Copper-free click chemistry is based on the reaction of strained cyclooctynes (such as BCN, DBCO) or cyclooctynes activated by electron-withdraing substituents (MOFO, DIFO) with azides in the absence of Cu catalyst at low temperature. The SPAAC (strain-promoted alkyne-azide click chemistry) reaction developed by Carolyn Bertozzi’s group can be applied for in vivo chemoselective ligation to biomolecules in the same manner as the Staudinger ligation (reaction between a phosphine and an azide with release of nitrogen), but with the advantage of a much more rapid reaction. Recent applications of Cu-free click chemistry to peptides include the synthesis of a DOTA-peptide conjugate prepared by the attachment of DOTA to MOFO followed by conjugation to an azide-modified α-MSH peptide. The resulting conjugate can form chelates with radionuclides for imaging applications such as tumor targeting. As the cyclooctynes vary considerably in reactivity, multiple SPAAC is feasible.
Subscribe to our newsletter
"*" indicates required fields
References
R. Huisgen
Kinetics and mechanism of 1,3-dipolar cycloadditions.
Angew. Chem. Int. Ed. 2, 633-645 (1963)
R. Huisgen
1,3-Dipolar cycloadditions – Introduction, survey, mechanism.
1,3-Dipolar Cycloaddition Chemistry (A. Padwa, ed), Wiley New York, 1-176 (1984)
H.C. Kolb et al.
Click chemistry: Diverse chemical function from a few good reactions.
Angew. Chem. Int. Ed. 40, 2004-2021 (2001)
V.V. Rostovtsev et al.
A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective ligation of azides and terminal alkynes.
Angew. Chem. Int. Ed. 41 2596–2599 (2002)
C.W. Tornøe et al.
Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides.
J. Org. Chem. 67, 3057–3064 (2002)
H.C. Kolb and K.B. Sharpless
The growing impact of click chemistry on drug discovery.
Drug Discov. Today 8, 1128-1137 (2003)
N.J. Agard et al.
A strain-promoted [3 + 2] azidealkyne cycloaddition for covalent modification of biomolecules in living systems.
J. Am. Chem. Soc. 126, 15046-15047 (2004)
W.S. Horne et al.
Heterocyclic peptide backbone modifications in an alpha-helical coiled coil.
J. Am. Chem. Soc. 126, 15366-15367 (2004)
A. Brik et al.
1,2,3-triazole as a peptide surrogate in the rapid synthesis of HIV-1 protease inhibitors.
Chembiochem 6, 1167-1169 (2005)
N.J. Agard et al.
A comparative study of bioorthogonal reactions with azides.
ACS Chem. Biol. 1, 644-648 (2006)
K. Oh and Z. Guan
A convergent synthesis of new betaturn mimics by click chemistry.
Chem. Commun. (Camb) 3069-3071 (2006)
J.M. Baskin et al.
Copper-free click chemistry for dynamic in vivo imaging.
Proc. Natl. Acad. Sci. U. S. A. 104, 16793-16797 (2007)
V.D. Bock et al.
1,2,3-Triazoles as peptide bond isosteres: synthesis and biological evaluation of cyclotetrapeptide mimics.
Org. Biomol. Chem. 5, 971-975 (2007)
L.K. Rasmussen et al.
Ruthenium-catalyzed cycloaddition of aryl azides and alkynes.
Org. Lett. 9, 5337-5339 (2007)
A. Tam et al.
Protein prosthesis: 1,5-disubstituted[1,2,3]triazoles as cis-peptide bond surrogates.
J. Am. Chem. Soc. 129, 12670-12671 (2007)
B.C. Boren et al.
Ruthenium-catalyzed azide-alkyne cycloaddition: scope and mechanism.
J. Am. Chem. Soc. 130, 8923-8930 (2008)
C.D. Hein et al.
Click chemistry, a powerful tool for pharmaceutical sciences.
Pharm. Res. 25, 2216-2230 (2008)
M. Meldal and C.W. Tornøe
Cu-catalyzed azide-alkyne cycloaddition.
Chem. Rev. 108, 2952–3015 (2008)
A. Le Chevalier Isaad et al.
Side chain-to-side chain cyclization by click reaction.
J. Pept. Sci. 15, 451-454 (2009)
D.C. Dieterich et al.
In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons.
Nat. Neurosci. 13, 897-905 (2010)
J. Dommerholt et al.
Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells.
Angew. Chem. Int. Ed. 49, 9422-9425 (2010)
M. Galibert et al.
Application of click-click chemistry to the synthesis of new multivalent RGD conjugates.
Org. Biomol. Chem. 8, 5133-5138 (2010)
J.C. Jewett and C.R. Bertozzi
Cu-free click cycloaddition reactions in chemical biology.
Chem. Soc. Rev. 39, 1272-1279 (2010)
C.B. Yim et al.
Synthesis of DOTA-conjugated multimeric [Tyr3]octreotide peptides via a combination of Cu(I)-catalyzed “click” cycloaddition and thio acid/ sulfonyl azide “sulfo-click” amida-tion and their in vivo evaluation.
J. Med. Chem. 53, 3944-3953 (2010)
M.F. Debets et al.
Bioconjugation with strained al-kenes and alkynes.
Acc. Chem. Res. 44, 805-815 (2011)
S. Richter et al.
Synthesis of neurotensin(8-13)-phosphopeptide heterodimers via click chemistry.
Bioorg. Med. Chem. Lett. 20, 3306-3309 (2010)
S.G. Agalave et al.
Click chemistry: 1,2,3-triazoles as pharmacophores.
Chem. Asian J. 6, 2696-2718 (2011)
E.M. Sletten and C.M.Bertozzi
From mechanism to mouse: a tale of two bioorthogonal reactions.
Acc. Chem. Res. 44, 666-676 (2011)
X. Li
Click to join peptides/proteins together.
Chem. Asian J. 6, 2606-2616 (2011)
M. Góngora-Benitez et al.
A universal strategy for preparing protected C-terminal peptides on the solid phase through an intra-molecular click chemistry-based handle.
Chem. Commun. (Camb) 48, 2313-2315 (2012)
J.M.Palomo
Click reactions in protein chemis-try: from the preparation of semisynthetic enzymes to new click enzymes.
Org. Biomol. Chem. 10, 9309-9318 (2012)
M. Wang et al. (2012)
“Click chemistry” for molecular imaging
Curr. Mol. Imaging 1, 87-95 (2012)
H.Li et al.
Click chemistry in peptide-based drug design.
Molecules 18, 9797-9817 (2013)
J. Thundimadathil
Click chemistry in peptide science: a mini-review. Synthesis of clickable peptides and applications.
Chimica Oggi -Chemistry Today 31, 34-37 (2013)
D. Zeng et al.
The growing impact of bioorthogonal click chemistry on the development of radiopharmaceuticals.
J. Nucl. Med. 54, 829-832 (2013)
J. Dommerholt et al.
Highly accelerated inverse electrondemand cycloaddition of electrondeficient azides with aliphatic cyclooctynes.
Nat. Commun. 5, 5378 (2014)
W. Tang and M.L.Becker
“Click” reactions: a versatile toolbox for the synthesis of peptide-conjugates.
Chem. Soc. Rev. 43, 7013-7039 (2014)
Y.H.Lau et al.
A two-component ‘double-click’ approach to peptide stapling.
Nat. Protoc. 10, 585-594 (2015)