PIONEERING PARTNER FOR COSMETIC PEPTIDES
Peptides are involved in many physiological processes. Their broad acceptance as natural molecules, relatively high stability and well-defined actions make them attractive for many skin-related indications, most notably in anti-aging therapy. We have considerable expertise and long-standing experience in peptide synthesis. With our capacity to upscale the production of simple and modified pep-tides, we are the partner of choice for the pharmaceutical and cosmetic industries.
English
Japanese
Chinese
Our portfolio reaches from catalog products, which can be purchased in small amounts for research, to the production of commercial batch sizes used by our customers for formulation. In addition, we offer several services such as the assurance of the compatibility with CMR requirements and microbiological limits.
| Step in project | Performed by |
|---|---|
| Catalog products (small amounts, mostly from stock) for early research | Bachem |
| R&D, discovery of new peptide cosmetic ingredients | Our Customers |
| Handling of intellectual properties on the active ingredients | Our Customers |
| Synthesis of the first proof of concept batch for early development | Bachem |
| Formulation development | Our Customers |
| Early tests on mode of action and dosage | Our Customers |
| Process development for scale-up till the commercial batch size | Bachem |
| Compatibility with CMR requirements (residual solvent, heavy metal traces) | Bachem |
| Compatibility with microbiological limits | Bachem |
| Toxicological studies | Our Customers |
| INCI registration | Our Customers |
| Production of commercial peptide batches | Bachem |
Introduction
The cosmetic industry requires a variety of different ingredients for skin care products. These include peptides, growth factors, antioxidants, anti-inflammatory botanicals, and polysaccharides. As these products show drug-like effects they are commonly referred to as cosmeceuticals. In contrast to drugs these ingredients are not regulated by the U.S. Food and Drug Administration (FDA). Originally, peptides became of interest in cosmetics as a result of the discovery of their beneficial effects in wound healing. As peptides are involved in an immense number of physiological processes, it was logical to further exploit them for cosmetic applications. Most of the peptides used in cosmetics are designed to counteract the aging process of the skin. The need for such products is driven by the increasing desire in modern society to maintain a young appearance even at an older age, and the breadth of possible treatments to achieve this goal. The increasing knowledge about the molecular details of the skin-related aging processes has significantly contributed to the exploration of novel anti-aging agents.
Skin Aging
Skin aging is a process influenced by extrinsic and intrinsic factors and is manifested by a progressive loss of skin tissue, the gradual loss of skin elasticity, and the appearance of fine lines and wrinkles. Extrinsic factors include exposure to UV-light, environmental pollution, cigarette smoke, and extreme weather conditions. The continuous generation of reactive oxygen species (ROS) during cellular metabolism and genetic predispositions are considered as intrinsic factors. Extrinsic factors, most notably UV irradiation and smoking, seem to be the major contributors to premature skin aging. Exposure of skin to UV-light increases ROS levels leading to changes in gene and protein structure and function and subsequent skin damage. Obvious effects of UV exposure are the disruption of ongoing collagen synthesis, induction of collagenase and other extracellular matrix protein-degrading enzymes, and the damage of cellular DNA. The premature skin-aging effects of tobacco smoking are caused by induction of collagenase activity and the reduced blood flow to the skin due to nicotine-induced vasoconstriction. Intrinsic factors such as the generation of ROS contribute to the loss of extracellular matrix proteins, a decrease in cutaneous blood flow, and a loss of cells and cell function.
Peptides in Cosmetics
Peptides have become very important ingredients in cosmetic products, especially in anti-aging preparations, and many of them are already available as research grade catalog products (Table 1). According to their mode of action, they have been divided into three main groups: signal peptides, neurotransmitter-affecting peptides, and carrier peptides. The first group mainly consists of peptides which are able to increase collagen synthesis, or alternatively, inhibit the breakdown of collagen by collagenase. The second group includes peptides mimicking the effects of botulinum neurotoxins whereas the third group, the carrier peptides, acts by delivering trace elements required for enzymatic processes.
Signal peptides
Aged skin is, amongst others, characterized by reduced levels of collagen and elastin. Increasing the number of fibroblasts or their collagen production and/or inhibiting further collagen hydrolysis are therefore considered effective means to halt or slow the aging process of the skin. Many of the peptides used in cosmetic preparations are compounds which act on fibroblasts. One of the peptides described to act in this way is H-Val- Gly-Val-Ala-Pro-Gly-OH (VGVAPG) (Product: 4010536 Chemotactic Domain of Elastin). 4010536 is an elastin-derived peptide sequence repeated several times in tropoelastin. It was found to stimulate the proliferation of human skin fibroblasts presumably via the elastin receptor.
The N-terminally palmitoylated peptide is marketed under the name of palmitoyloligopeptide and is supposed to penetrate more efficiently through the epidermis than the parent compound. H-Lys-Thr-Thr-Lys-Ser-OH (KTTKS) (Product: 4025012 Procollagen Type I (212-216)) a subfragment of the carboxy-terminal propeptide of type I collagen (residues 197-241) and represents the minimal sequence shown to stimulate extracellular matrix biosynthesis in fibroblasts. It augments type I and II collagen and fibronectin production in a dose- and time-dependent manner with no effect on total protein synthesis or on the ratio of secreted proteins to cell-associated proteins.
The N-terminally palmitoylated peptide is marketed as a cosmetic ingredient under the designation palmitoyl pentapeptide-3. Another peptide shown to enhance collagen production when added to cultured fibroblasts is H-Gly-His- Lys-OH, also known as GHK (Product: 4000308 Liver Cell Growth Factor). Since similar enhancement of collagen synthesis was shown for the tripeptide in complex with copper (see below) it was not clear whether the activity was intrinsic to GHK or whether it was resulting from the formation of active copper complexes due to the presence of copper ions in the tissue culture medium.
As various matrix metalloproteinases are involved in the degradation of collagen and elastin, inhibition of these enzymes represents another strategy to prevent extracellular matrix breakdown and its influence on skin aging. Several peptides have been shown to act in this way: H-Tyr-Tyr-Arg-Ala- Asp-Asp-Ala-OH (YYRADDA) corresponds to a sequence in α-1 type I procollagen which is cleaved during the conversion of procollagen to collagen. The peptide was shown to attenuate collagen breakdown by inhibiting procollagen-C proteinase, which cleaves the C-propeptide from type I procollagen. Another compound, the tripeptide H-Lys-Phe-Lys-OH (Product: 4000074) linked to elaidic acid, was demonstrated to activate TGF-β, a factor showing the capacity to increase collagen, elastin and TIMP-1 (tissue inhibitor of metalloproteinase 1) expression, and to inhibit MMPs via its lipophilic moiety, elaidic acid.
The sequence of H-Lys-Phe-Lys-OH corresponds to the consensus BFB (B: basic amino acid, F: phenylalanine) shown to activate LAP-TGF-β in several in vitro and ex vivo studies. Elaidic acid was chosen due to its inhibitory effect on gelatinase A (MMP-2) and B (MMP-9). Similar effects on TGF-β were shown for the palmitoylated peptide Palmitoyl-Lys- Val-Lys-OH and Palmitoyl-Val-Gly-Val-Val-Ala-Pro-Gly-OH, an elastin-derived peptide.
| Prod. No. | Product | Activity |
|---|---|---|
| 4012417 | Ac-Hyp-OH | Anti-inflammatory, wound healing |
| 4003668 | Ac-Met-OH | Anti-wrinkle |
| 4016348 | Ac-Ser-Asp-Lys-Pro-OH | Stimulation of keratinocyte, fibroblast and follicle dermal papilla cell growth |
| 4001968 | Ac-Tyr-NH₂ | Anti-wrinkle |
| 4012005 | H-Ala-His-Lys-OH | Enhancement of collagen synthesis |
| 4027810 | L-Anserine · nitrate | Antioxidant |
| 4026204 | Carcinine | Antioxidant |
| 4030364 | L-Carnosine | Antioxidant |
| 4010536 | Chemotactic Domain of Elastin (VGVAPG) | Stimulation of skin fibroblast proliferation |
| 4038287 | rec β-Defensin 2 (human) | Elevated expression in psoriasis and in the inflamed skin of mastitis |
| 4061590 | Dermcidin-1L (human) | Activation of keratinocytes |
| 4030547 | H-α-Difluoro-Me-DL-Orn-OH · HCl · H₂O | Reduction of excessive hair growth (hirsutism) |
| 4003792 | (D-Ala²)-Leu-Enkephalin | Anti-wrinkle peptide |
| 4008512 | H-Gly-Pro-Hyp-OH | Anti-wrinkle and anti-aging |
| 4009573 | Z-Gly-Pro-Phe-Pro-Leu-OH | Inhibitor of desquamation of human skin |
| 4030783 | rec IL-1α (human) | Role in skin renewal |
| 4026408 | Z-Ile-Glu(OtBu)-Ala-Leu-aldehyde | Inhibitor of accumulation of ubiquitinylated proteins in neuronal cells |
| 4003049 | Kyotorphin | Reducing the sensitivity of the skin |
| 4000308 | Liver Cell Growth Factor (GHK) | Enhancement of collagen synthesis |
| 4000074 | H-Lys-Phe-Lys-OH (KFK) | Activation of LAP-TGF-? |
| 4003800 | (Nle⁴,D-Phe⁷)-α-MSH | Anti-inflammatory |
| 4026419 | (Met⁵,Pro⁶,D-Phe⁷,D-Trp⁹,Phe¹⁰)-α-MSH (5-13) | Anti-inflammatory |
| 4006104 | α-MSH (11-13) (free acid) | Anti-inflammatory |
| 4011677 | (D-Pro¹²)-α-MSH (11-13) (free acid) | Anti-inflammatory |
| 4001965 | H-Phe-β-Ala-OH | Inhibitor of hair growth. Eventually promoting effect on wound healing |
| 4025012 | Procollagen Type I (212-216) (KTTKS) | Enhancement of collagen synthesis |
| 4001630 | H-Pro-Hyp-OH | Stimulation of fibroblast proliferation, chondroprotective |
| 4030572 | rec EGF (human) | Aiding wound healing |
| 4011277 | Rigin (GQPR) | Immunomodulation |
| 4026105 | SPARC (119-122) (mouse) | Stimulation of endothelial cell proliferation and angiogenesis |
| 4043020 | Thymosin β₄ (human, bovine, horse, rat) | Potentially contributing to angiogenesis, wound healing, regulation of inflammation and other processes |
| 4008196 | H-Val-Trp-OH | Compound in various formulations, e.g. for anti-wrinkle and anti-aging cosmetics |
| 4001260 | H-Val-Tyr-Val-OH | Peptidic compound in various cosmetic formulations |
Table 1: Cosmeceutical-related research quantities offered from our catalog
Neurotransmitter-affecting peptides
Many of the peptides used in cosmetic preparations belong to the group of neurotransmitter-affecting peptides. These peptides act in a similar way as botulinum toxin (Botox). By inhibiting signal transduction pathways at neuromuscular junctions (Figure 1) they attenuate the formation of wrinkles and fine lines which appear over time due to the repetitive contraction of the intrinsic muscles of facial expression. Botulinum toxin, synthesized by the bacterium Clostridium botulinum, is the most potent toxin known. It is a disulfide-linked heterodimer consisting of a heavy and a light chain.
Upon binding to the peripheral neuronal presynaptic membrane mediated by the heavy chain the toxin is internalized by receptor-mediated endocytosis. After translocation from the endocytotic vesicle into the cytoplasm, the light chain proteolytically cleaves either SNAP-25 or synaptobrevin depending on the serological subtype of the neurotoxin. Cleavage of these proteins which are essential for docking to and fusion of acetylcholine vesicles with the inner side of the nerve terminal membrane results in the inhibition of neurotransmitter release at neuromuscular junctions. A well-known mimic of botulinum toxin used in cosmetic preparations is Acetyl-Glu-Glu-Met-Gln-Arg-Arg-NH2. It is a synthetic peptide corresponding to a sequence within the N-terminal region of SNAP-25 (amino acids 12-17) and competes with SNAP-25 for a position in the SNARE complex, thereby modulating its formation.
The resulting destabilization of the SNARE complex leads to an inhibition of neurotransmitter release and a subsequent attenuation of muscle contraction. Another class of potentially interesting substances in the cosmetic industry includes peptides derived from snake venoms. Waglerin-1 (H-Gly-Gly-Lys-Pro-Asp-Leu-Arg-Pro-Cys-His-Pro-Pro-Cys-His-Tyr-Ile-Pro-Arg-Pro-Lys-Pro-Arg-OH), for example, is a peptide isolated from the venom of the temple viper, Tropidolaemus wagleri. It consists of 22 amino acids and selectively blocks the epsilon form of the muscular nicotinic acetylcholine receptor (mnAChR) thereby keeping the affected muscles in a relaxed state.
Another compound, the tripeptide H-β-Ala-Pro-Dab-NHBzl mimicking the activity of waglerin-1 is now marketed in anti-wrinkle creams. Many other peptides acting similarly by affecting neurotransmitter release are promoted by the cosmetic industry. Compounds include: a) a pentapeptide, which binds to the enkephalin receptors on the outside of neurons and thereby initiates a cascade leading to a decrease in acetylcholine release and subsequent muscle contraction; b) SNAP-8, an octapeptide related to and acting in the same way as Acetyl-Glu-Glu- Met-Gln-Arg-Arg-NH2; and c) a hexapeptide that reduces the activation of muscle specific kinase (MuSK) by blocking the agrin binding site.
The peptide was to disrupt acetylcholine receptor clustering, a requirement for acetylcholine to trigger the signal for muscle contraction.
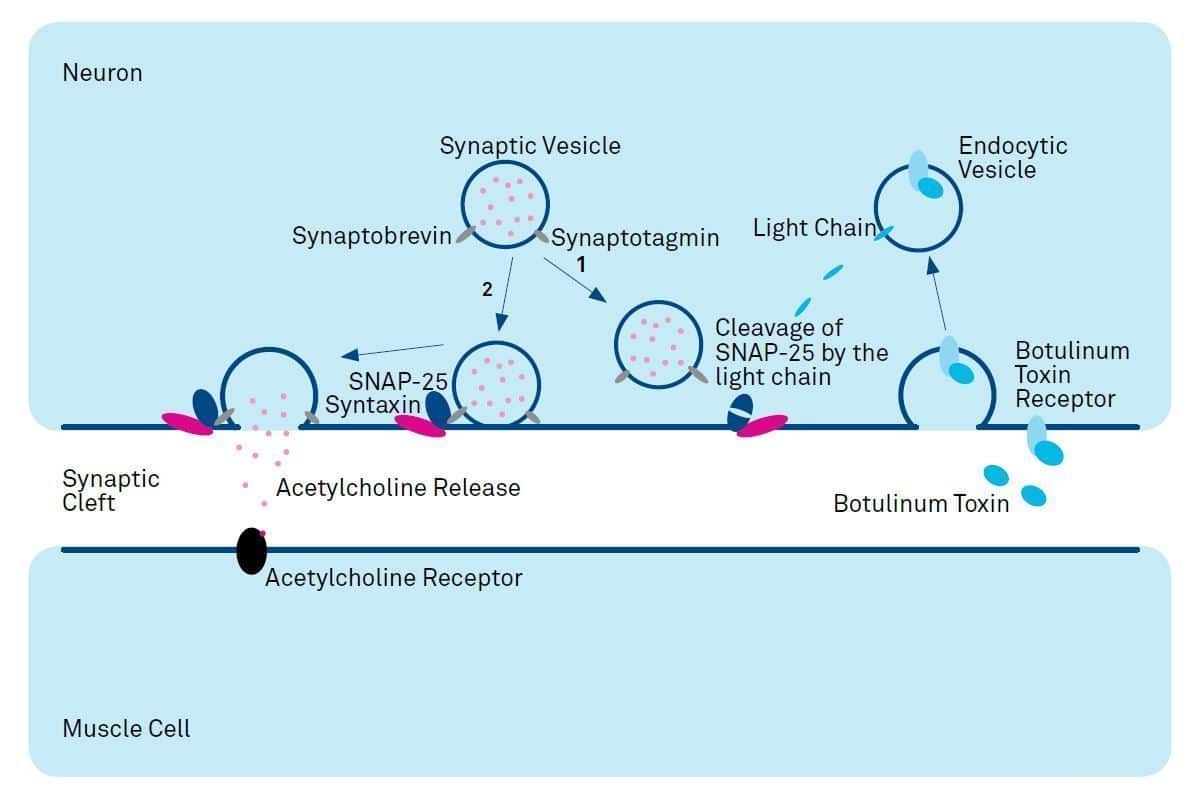
Figure1:
Mode of Action of Botulinum Neurotoxin Type A Botulinum Neurotoxin Type A consists of a heavy and a light chain. Upon binding to the botulinum toxin receptor the toxin is internalized. Acidification of the vesicles leads to the translocation of the light chain to the cytosol.
There it acts as a protease and cleaves the SNARE protein SNAP- 25. Cleavage results in inhibition of exocytosis and acetylcholine neurotransmitter release (1) as intact SNAP-25 together with several other proteins is required for synaptic vesicle fusion with the presynaptic membrane (2).
Carrier peptides
The tripeptide H-Gly-His-Lys-OH (GHK) (Product: 4000308 Liver Cell Growth Factor) was originally identified in human plasma and has a high affinity for copper2+ (Cu2+). It acts as a signaling peptide and a carrier molecule for copper which is a co-factor for several enzymes involved in collagen and elastin formation. The copper peptide was shown to stimulate wound healing but also to reduce fine lines and wrinkles and to improve elasticity and firmness of aged skin. A wide variety of effects have been ascribed to GHK-Cu. The peptide exhibits anti-inflammatory actions by suppressing the expression of pro-inflammatory cytokines. It also chemoattracts capillary cells, macrophages and mast cells, increases the synthesis of collagen and elastin, and stimulates the proliferation of fibroblasts and keratinocytes.
Other mechanisms
Many peptides used in cosmetic preparations act by other mechanisms to improve skin appearance or delay skin aging. These include ROS scavengers, collagen fiber organizing compounds, and anti-inflammatory peptides: Peptides such as carnosine (Product: 4030364 ), anserine (Product: 4027810 L-Anserine · nitrate) and carcinine (Product: 4026204) are histidine dipepeptides with antioxidant activity. Carnosine has been shown to scavenge ROS and chelate prooxidative metals. It also inactivates reactive mono- and dialdehydes released during the oxidative breakdown of unsaturated lipids thereby protecting hydrophilic and lipophilic biological molecules from oxidative damage.
However, in contrast to carcinine it is sensitive to enzymatic hydrolysis by carnosinase. A tetrapeptide of unpublished sequence is a mimic of decorin which interacts with collagen and influences collagen fibrillogenesis. As functional decorin in the skin diminishes with age, the tetrapeptide is proposed to serve as a substitute. By controlling collagen aggregation and homogenization of fibril diameters and dimensions, it increases skin suppleness and thus improving its appearance. Rigin (Product: 4011277 H-Gly-Gln-Pro-Arg-OH (GQPR)) corresponds to amino acid sequence 341- 344 of the human IgG H-chain and has been shown to possess immunomodulatory activity. By suppressing inflammation the palmitoylated form is supposed to accelerate tissue repair thereby leading to increased skin firmness, smoothness, and elasticity.
| Custom Synthesis at Bachem |
|---|
| ✔ QUALITY |
| • GMP and non-GMP quality |
| • Broad range of purities |
| • State of the art analytical capabilities |
| • ISO 13485 certified manufacturing site in Vista, USA |
| ✔ CHEMISTRY |
| • Fmoc-, Boc-, Z- and other synthetic strategies |
| • Native chemical ligation |
| • Synthesis of complex peptides |
| ✔ CAPACITY |
| • Production sites in the USA and Europe |
| • Largest production facilities in the market |
| • Up-to-date technology |
| ✔ MODIFICATIONS |
| • Acylation, acetylation, amidation, etc. |
| • Cyclizations |
| • Stabilizing modifications |
| ✔ SUPPORT |
| • Highly qualified technical support team |
| • Documentation |
| • Confidentiality |
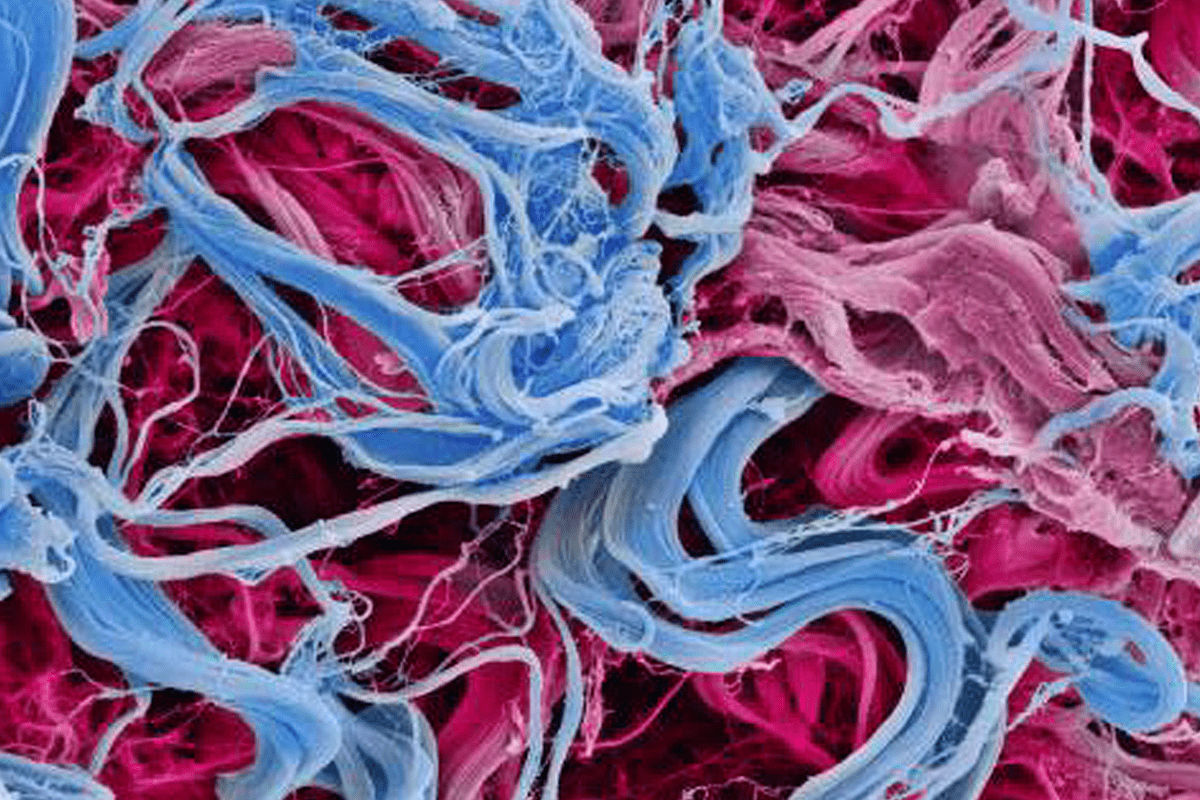
COLLAGEN FIBRES
Colored scanning electron micrograph (SEM) of collagen from the dermis of the skin.
Collagen is a protein with a high tensile strength, providing structure and elasticity to skin, tendons, ligaments and bones. It is the most abundant protein in the body. In the dermis, collagen forms rope-like fibers that are arranged irregularly.
Conclusions
Synthetic peptides have become important for the cosmetic industry. Due to demographic changes and the aging population wishing to maintain a young lifestyle and appearance, cosmetic industry research has focused on anti-aging skin therapy.
Today, more than 30 peptides are used in anti-aging skin care products and there are many more in development. Bachem is the market leader in the production of synthetic peptides and has long standing partnerships with major firms in the pharmaceutical and cosmetic industry.
We are well-equipped for small to industrial scale synthesis of peptides of any complexity. Bachem is therefore the ideal partner in the development and production of cosmetic peptides. Together with our customers, we accomplish each of the demanding steps in a development project, finally leading to the successful cosmetic product.
Subscribe to our newsletter
"*" indicates required fields
References
L. Pickart et al.
Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells.
Nature 288, 715-717 (1980)
F.K. Njieha et al.
Partial purification of a procollagen C-proteinase. Inhibition by synthetic peptides and sequential cleavage of type I procollagen.
Biochemistry 21, 757-764 (1982)
C.M. Perkins et al.
The structure of a copper complex of the growth factor glycyl-L-histidyl-L-lysine at 1.1 Å resolution.
Inorg. Chim. Acta 82, 93-99 (1984)
R. Rocchi et al.
Synthesis and biological activity of tuftsin and rigin derivatives containing monosaccharides or monosaccharide derivatives.
Int. J. Pept. Protein Res. 29, 262-275 (1987)
K. Katayama et al.
Regulation of extracellular matrix production by chemically synthesized subfragments of type I collagen carboxy propeptide.
Biochemistry 30, 7097-7104 (1991)
K. Katayama et al.
A pentapeptide from type I procollagen promotes extracellular matrix production.
J. Biol. Chem. 268, 9941-9944 (1993)
M.A. Babizhayev et al.
L-carnosine (beta-alanyl-L-histidine) and carcinine (beta-alanylhistamine) act as natural antioxidants with hydroxyl-radicalscavenging and lipid-peroxidase activities.
Biochem. J. 304 (Pt 2), 509-516 (1994)
A. Kamoun et al.
Growth stimulation of human skin fibroblasts by elastin-derived peptides.
Cell Adhes. Commun. 3, 273-281 (1995)
S. Schultz-Cherry et al.
Regulation of transforming growth factorbeta activation by discrete sequences of thrombospondin 1.
J. Biol. Chem. 270, 7304-7310 (1995)
L.M. Gutierrez et al.
A peptide that mimics the C-terminal sequence of SNAP-25 inhibits secretory vesicle docking in chromaffin cells.
J. Biol. Chem. 272, 2634-2639 (1997)
A.R. Hipkiss
Carnosine, a protective, anti-ageing peptide?
Int. J. Biochem. Cell. Biol. 30, 863-868 (1998)
J.J. McArdle et al.
Waglerin-1 selectively blocks the epsilon form of the muscle nicotinic acetylcholine receptor.
J. Pharmacol. Exp. Ther. 289, 543-550 (1999)
K. Lintner and O. Peschard
Biologically active peptides: from a laboratory bench curiosity to a functional skin care product.
Int. J. Cosmet. Sci. 22, 207-218 (2000)
A. Berton et al.
Involvement of fibronectin type II repeats in the efficient inhibition of gelatinases A and B by long-chain unsaturated fatty acids.
J. Biol. Chem. 276, 20458-20465 (2001)
C. Blanes-Mira et al.
A synthetic hexapeptide (Argireline) with antiwrinkle activity.
Int. J. Cosmetic Sci. 24, 303-310 (2002)
E.R. Chapman
Synaptotagmin: a Ca2+ sensor that triggers exocytosis?
Nat. Rev. Mol. Cell Biol. 3, 498-508 (2002)
J.H. Cauchard et al.
Activation of latent transforming growth factor beta 1 and inhibition of matrix metalloprotease activity by a thrombospondinlike tripeptide linked to elaidic acid.
Biochem. Pharmacol. 67, 2013-2022 (2004)
C. Montecucco et al.
SNARE complexes and neuroexocytosis: how many, how close?
Trends Biochem. Sci. 30, 367-372 (2005)
C.M. Choi and D.S. Berson
Cosmeceuticals.
Semin. Cutan. Med. Surg. 25, 163-168 (2006)
M.A. Babizhayev
Biological activities of the natural imidazole-containing peptidomimetics n-acetylcarnosine, carcinine and L-carnosine in ophthalmic and skin care products.
Life Sci. 78, 2343-2357 (2006)
M.P. Lupo and A.L. Cole
Cosmeceutical peptides.
Dermatol. Ther. 20, 343-349 (2007)
T.M. Callaghan and K.P. Wilhelm
A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing.
Int. J. Cosmet. Sci. 30, 313-322 (2008)
F.J. Erbguth
From poison to remedy: the chequered history of botulinum toxin.
J. Neural Transm. 115, 559-565 (2008)
L. Pickart
The human tri-peptide GHK and tissue remodeling.
J. Biomater. Sci. Polym. Ed. 19, 969-988 (2008)
A. Puig et al.
A new decorin-like tetrapeptide for optimal organization of collagen fibres.
Int. J. Cosmet. Sci. 30, 97-104 (2008)
S. Sikorra et al.
Substrate recognition mechanism of VAMP/synaptobrevin-cleaving clostridial neurotoxins.
J. Biol. Chem. 283, 21145-21152 (2008)
M. Amer and M. Maged
Cosmeceuticals versus pharmaceuticals.
Clin. Dermatol. 27, 428-430 (2009)
D.L. Bissett
Common cosmeceuticals.
Clin. Dermatol. 27, 435-445 (2009)
J.P. Preetha and K. Karthika
Cosmeceuticals – An evolution.
Int. J. ChemTech Res. 1, 1217-1223 (2009)
L. Robert et al.
Physiology of skin aging.
Pathol. Biol. (Paris) 57, 336-341 (2009)
L. Zhang and T.J. Falla
Cosmeceuticals and peptides.
Clin. Dermatol. 27, 485-494 (2009)
V.V. Pai et al.
Topical peptides as cosmeceuticals.
Indian J. Dermatol. Venereol. Leprol., 83(1), 9-18 (2017)