Pseudoproline and Isoacyl dipeptides offered by Bachem
Temporary Pro mimics can be readily obtained from Ser and Thr by oxazolidine or from Cys by thiazolidine (“pseudoproline”) formation. Both 2,2 dimethyloxazolidines and -thiazolidines are smoothly cleaved by trifluoroacetic acid and thus suitable for Fmoc-SPPS. Disruption of Ser- and / or Thr containing aggregates is achieved as well by introducing depsipeptide (“O-acyl isopeptide”) bonds into the peptide backbone. The resulting isopeptide is rearranged yielding the desired sequence in slightly basic solution.
Preformed Dipeptides
For facilitating the introduction of oxazolidine or thiazolidine moieties or isopeptide bonds during solid-phase synthesis, protected pseudoproline dipeptides and O-acyl dipeptides have been developed. We offer a broad choice of these versatile building blocks for preventing aggregation during Fmoc-SPPS.
Pseudoproline Dipeptides
Stepwise Fmoc-SPPS may become very difficult or even fail if the resin-bound peptide aggregates. Unfortunately, the predictions of “difficult sequences” based on an assumed propensity of the sequence to form β-sheets are not very reliable, with the exception that such aggregates cannot be formed in the vicinity of proline. The induction of a cis-amide bond by Pro disrupts β-sheets as well as α-helices.
The aggregation of Ser-, Thr- or Cys-containing peptides during Fmoc-SPPS can thus be efficiently prevented by introducing the Fmoc-protected oxazolidines or thiazolidines, which can be obtained from these amino acids and aldehydes or ketones (Fig. 1). These heterocycles have been appropriately termed pseudoprolines. The pseudoproline approach, originally developed by Manfred Mutter et al. at EPFL Lausanne1T. Haack and M. Mutter
Serine derived oxazolidines as secondary structure disrupting, solubilizing building blocks in peptide synthesis. Tetrahedron Lett. 33, 1589-1592 (1992)2M. Mutter et al. Pseudo-prolines (psiPro) for accessing “inaccessible” peptides. Pept. Res. 8, 145-153 (1995), usually employed the Ser and Thr derivatives, as the oxazolidines were cleaved more readily by acids than the thiazolidines3T. Wöhr et al. Pseudoprolines as a solubilizing, structuredisrupting protection technique in peptide synthesis. J. Am. Chem. Soc. 118, 9218-9227 (1996).
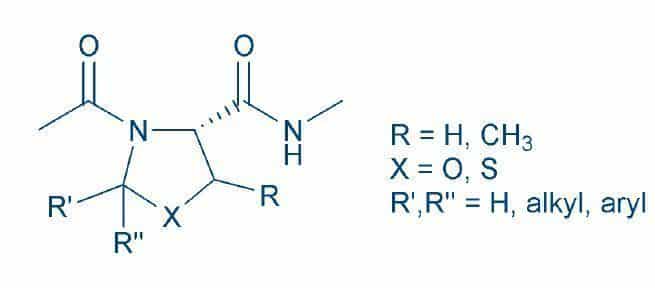
Fig. 1
In the meantime, thiazolidine cleavage conditions suitable for standard Fmoc-SPPS have been developed 4T.M. Postma and F. Albericio
Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in Fmoc-based solid-phase peptide synthesis, Org. Lett. 16, 1772-1775 (2014). The incorporation of cysteine pseudoproline derivatives facilitated the synthesis of Cys-rich peptides5S. Chierici et al. A case study of 2,2 dimethylthiazolidine as locked cis proline amide bond: synthesis, NMR and molecular modeling studies of a delta-conotoxin EVIA peptide analog. Org. Biomol. Chem. 2, 2437-2441 (2004), which will also benefit from milder deprotection conditions. The acid lability of both types of heterocycle can be fine-tuned by the choice of substituents at the 2-position.
The isopropylidene group of the oxazolidine (X = O, R’, R’’ = CH3) proved to be the best choice for Fmoc-SPPS 6T. Wöhr et al. Pseudoprolines as a solubilizing, structuredisrupting protection technique in peptide synthesis. J. Am. Chem. Soc. 118, 9218-9227 (1996), as the cycle is opened readily yielding Ser (R = H) or Thr (R = CH3) during the final cleavage with TFA. Both isopropylidene (X = S, R’, R’’ = CH3) and benzylidene (X = S, R’= 2,4-Dimethoxyphenyl, R’’ = H) derivatives have been used for incorporating cysteine, as the rings can be cleaved by TFA7T.M. Postma and F. Albericio
Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in Fmoc-based solid-phase peptide synthesis Org. Lett. 16, 1772-1775 (2014).
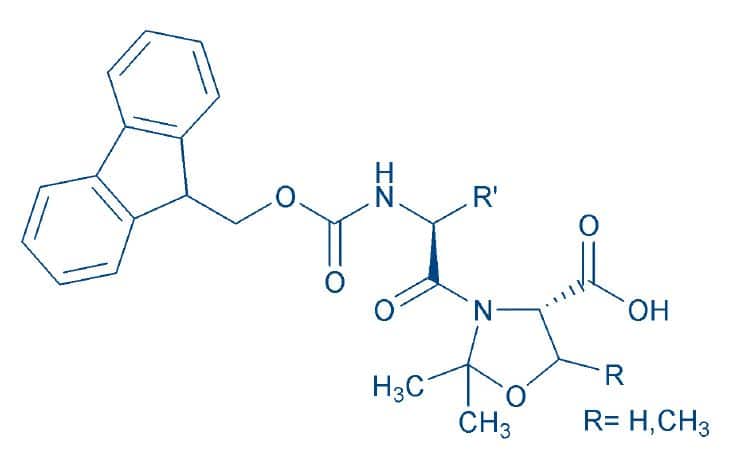
Fig. 2
The main drawback of this approach is the difficult coupling of the following amino acid to the hindered heterocycle. To circumvent this, Fmoc pseudoproline dipeptides were introduced8T. Wöhr and M. Mutter, Pseudo-prolines in peptide synthesis:Direct insertion of serine and threonine derived oxazolidines in dipeptides. Tetrahedron Lett. 36, 3847-3848 (1995)(Fig. 2). The coupling of these building blocks represents the most straightforward method to incorporate pseudoprolines. The ”preventive” insertion of such moieties is highly recommended when synthesizing long peptides lacking prolines (Fig. 3).
The incorporation of pseudoprolines facilitates cyclization of peptides
The repeated inclusion of pseudoproline units during the elongation of the peptide will improve the overall coupling efficiency, even if aggregation does not pose a severe problem. A considerable number of syntheses of long or “inaccessible” peptides, which succeeded only due to the insertion of pseudoprolines in appropriate positions, has been published since the introduction of these derivatives 9P. White et al. Expediting the Fmoc solid phase synthesis of long peptides through the application of dimethyloxazolidine dipeptides. J. Pept. Sci. 10, 18-26 (2004) 10W.R. Sampson et al. The synthesis of „difficult“ peptides using 2-hydroxy-4-methoxybenzyl or pseudoproline amino acid building blocks: a comparative study. J. Pept. Sci. 5, 403-409 (1999)11M. Keller and A.D. Miller Access to the inaccessible sequence of cpn 60.1 (195-217A. Abedini and D.P. Raleigh Incorporation of pseudoproline derivatives) by temporary oxazolidine protection of selected amide bonds. Bioorg. Med. Chem. Lett. 11, 857-859 (2001). Difficult peptides as IAPP 12A. Abedini and D.P. Raleigh, Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregationprone polypeptide.
Org. Lett. 7, 693-696 (2005)13K. Page et al. Fast Fmoc synthesis of hAmylin1-37 with pseudoproline assisted on-resin disulfide formation.
J. Pept. Sci. 13, 833-838 (2007) or RANTES 14F. García-Martin et al. The synergy of ChemMatrix resin and pseudoproline building blocks renders RANTES, a complex aggregated chemokine. Biopolymers 84, 566-575 (2006)could be synthesized following standard Fmoc-SPPS protocols after evaluation of the required number and optimal position of the oxazolidine moieties to be inserted.
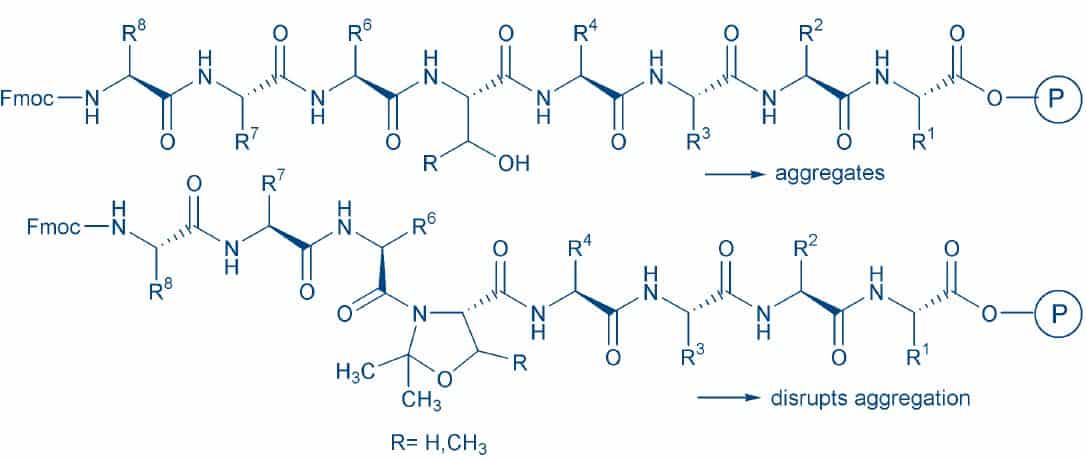
Fig. 3
As with Fmoc amino acid derivatives, couplings can be accelerated by microwave irradiation 15P. Marek et al. Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids. Org. Lett. 12, 4848-4851 (2010)16S. Northfield et al. Synthesis of “Difficult” Fluorescence Quenched Substrates of Granzyme C. Int. J Pept. Res. Ther. 16, 159-165 (2010)17P.W.R. Harris et al. A single pseudoproline and microwave solid phase peptide synthesis facilitates an efficient synthesis of human amylin 1-37. Int. J. Pept. Res. Ther. 19, 147-155 (2013). Pseudoproline dipeptides show their high versatility as building blocks not only during the SPPS of long and difficult peptides. When synthesizing short peptides, a distinctly purer crude product may be obtained by incorporating merely a single pseudoproline unit 18S. Northfield et al. Synthesis of “Difficult” Fluorescence Quenched Substrates of Granzyme C. Int. J Pept. Res. Ther. 16, 159-165 (2010).
The heterocycles are left intact when cleaving fully protected peptide fragments from SASRIN or 2-chlorotrityl resin with diluted TFA 19C. Sager et al. Influence of cis-trans isomerisation on pentapeptide cyclisation. Tetrahedron Lett. 40, 7987-7892 (1999)20C. Sager et al. Influence of cis-trans isomerisation on pentapeptide cyclisation. Tetrahedron Lett. 40, 7987-7892 (1999) and their presence markedly increases the solubility of the cleavage products. Accordingly, the purification and coupling of the fragments as well as the modification of partially protected peptides in solution are facilitated by insertion of pseudoproline moieties. As fragments containing a C-terminal proline, fragments with a C-terminal pseudoproline can be coupled with minimal concomitant racemization.
Hence pseudoproline dipeptides may establish additional possibilities in convergent peptide synthesis 21I. Coin et al. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. 14, 299-306 (2008). The incorporation of an oxazolidine moiety greatly facilitates cyclizations of Ser- or Thr-containing peptides, disulfide bridge formations 22K. Page et al. Fast Fmoc synthesis of hAmylin1-37 with pseudoproline assisted on-resin disulfide formation. J. Pept. Sci. 13, 833-838 (2007), as well as headto-tail cyclizations23D. Skropeta et al. Pseudoprolines as removable turn inducers: tools for the cyclization of small peptides. J. Org. Chem. 69, 8804-8809 (2004)24N. Schmiedeberg and H. Kessler Reversible backbone protection enables combinatorial solid-phase ring-closing metathesis reaction (RCM) in peptides. Org. Lett. 4, 59-62 (2002). The increased tendency to cyclize is due to the presence of a temporary cis-amide bond in the molecule 25P. Dumy et al. Pseudo-prolines as a molecular hinge: reversible induction of cis amide bonds into peptide backbones. J. Am. Chem. Soc. 119, 918-925 (1997)26M. Keller et al. Pseudoproline-containing analogues of morphiceptin and endomorphin-2: evidence for a cis Tyr-Pro amide bond in the bioactive conformation. J. Med. Chem. 44, 3896-3903 (2001).
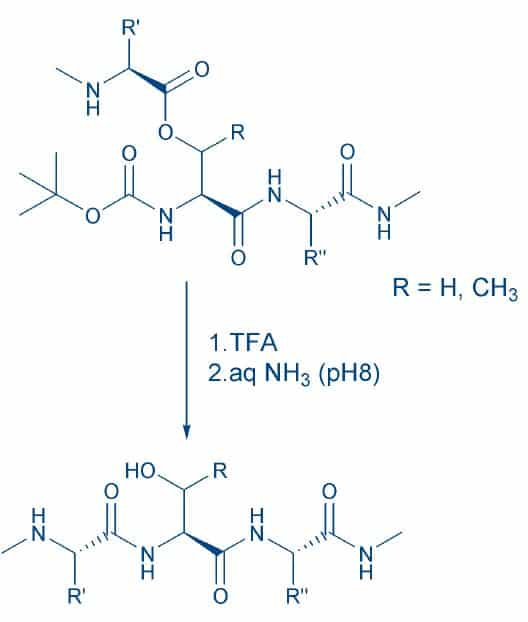
Fig. 4
Even though Cys occurs only rarely in peptides and proteins, far less often than Ser and Thr, the incorporation of cysteine pseudoproline is highly attractive due to the peculiar properties of the amino acid. Postma and Albericio showed that on-resin macrocyclization of Cys-containing peptides proceeds more smoothly if Cys(Trt) is replaced by a cysteine pseudoproline27T.M. Postma and F. Albericio Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in Fmoc-based solid-phase peptide synthesis Org. Lett. 16, 1772-1775 (2014).
They also observed that cysteine 2,2-dimethylthiazolidines are opened during the final cleavage with TFA/TIS/H2 O (95:2.5:2.5), lows avoiding this risk, an advantage worth considering when synthesizing multiple disulfide bridge-containing peptides or anchoring cysteine to carriers. If its thiazolidine ring is left intact, 2,2-dimethylthiazolidin-4-carboxylic acid (Me2 Thz), conveniently introduced as pseudoproline dipeptide, acts as a highly effective cis-proline mimic [24,25] 28C. Sager et al. Influence of cis-trans isomerisation on pentapeptide cyclisation. Tetrahedron Lett. 40, 7987-7892 (1999)29A. Wittelsberger et al. Introduction of a cis-prolyl mimic in position 7 of the peptide hormone oxytocin does not result in antagonistic activity. J. Med. Chem. 48, 6553-6562 (2005)30Z. Qian et al. Structure-based optimization of a peptidyl inhibitor against calcineurin-nuclear factor of activated T cell (NFAT) interaction. J. Med. Chem. 57, 7792-7797 (2014). An analog of the cyclopeptide phakellistatin 19 containing three Me2 Thz residues replacing proline, all of them incorporated by coupling pseudoproline dipeptides, showed enhanced activity 31M. Pelay-Gimeno et al. Rescuing biological activity from synthetic phakellistatin 19. J. Med. Chem. 56, 9780-9788 (2013). For synthesizing peptides containing this proline surrogate the standard TFA-labile lateral protecting groups may have to be replaced by highly acid-labile moieties such as Mtt (His), Trt (Ser) or OPp (Glu)32Z. Qian et al. Structure-based optimization of a peptidyl inhibitor against calcineurin-nuclear factor of activated T cell (NFAT) interaction.
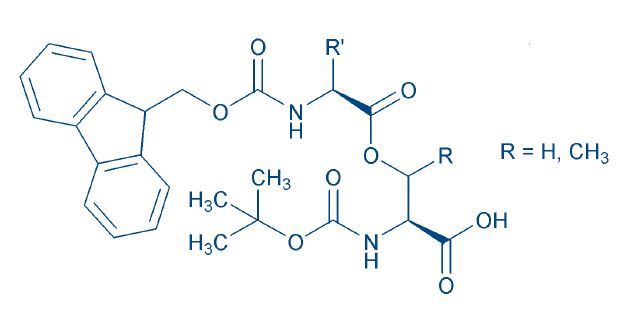
Fig. 5
Isoacyl Dipeptides
A major disadvantage of the pseudoproline approach cannot be left unmentioned: The solubilizing effect of the oxazolidine moiety is lost when deblocking the peptide with TFA. Albeit the quality of the crude product may be vastly improved, further purification will be tedious due to its low solubility. The N-O shift, a notorious side reaction during HF cleavage, turned out to be the key to the solution of this dilemma. This acid-catalyzed rearrangement involving the hydroxyl moiety of Ser or Thr residues can be smoothly reversed by keeping the peptide in a slightly basic medium (Fig. 4).
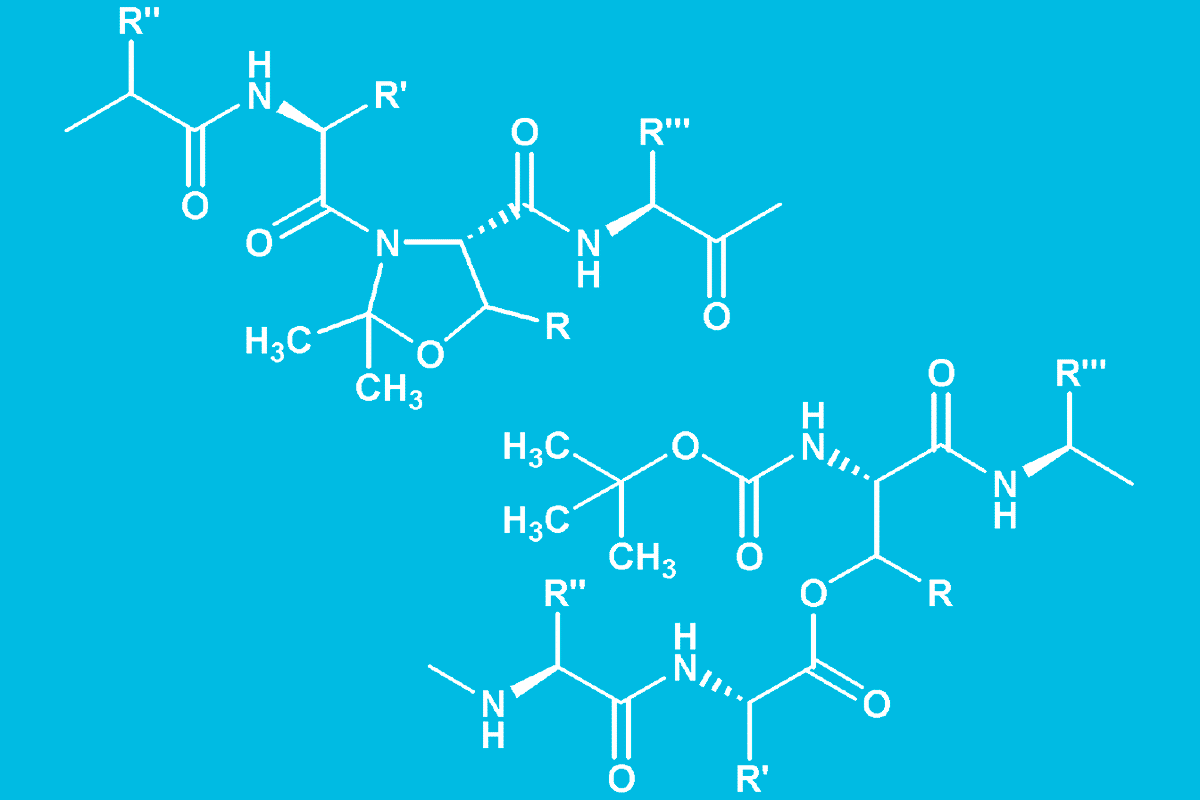
As the N-O shift, i.e. O-isoacyl peptide formation, causes a disruption of the secondary structure, it is accompanied by an increase in solubility33Y. Sohma et al. O-N intramolecular acyl migration reaction in the development of prodrugs and the synthesis of difficult sequence-containing bioactive peptides. Biopolymers 76, 344-356 (2004)34S. Dos Santos et al. Switch-peptides: controlling self-assembly of amyloid beta-derived peptides in vitro by consecutive triggering of acyl migrations. J. Am. Chem. Soc. 127, 11888-11889 (2005). At first, this type of depsipeptide was obtained by FmocSPPS involving on-resin esterification of the subsequent Fmoc-amino acid to the free hydroxyl moiety of an N-terminal Boc-Ser/Thr followed by elongation of the peptide 35Y. Sohma et al. Development of O-acyl isopeptide method. Biopolymers 88, 253-262 (2007). Low conversions, concomitant racemization, and diketopiperazine formation during the subsequent SPPS cycle 36I. Coin et al. Depsipeptide methodology for solid-phase peptide synthesis: circumventing side reactions and development of an automated technique via depsidipeptide units. J. Org. Chem. 71, 6171-6177 (2006) are the main drawbacks of this straightforward approach.
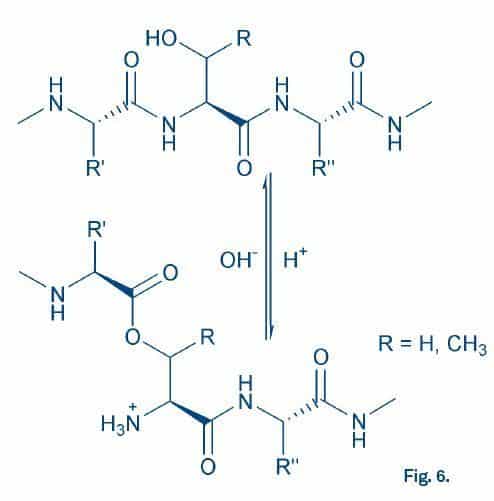
Fig. 6
The recently introduced Fmoc O-acyl dipeptides help to overcome these problems 37T. Yoshiya et al. „O-Acyl isopeptide method“ for peptide synthesis: synthesis of forty kinds of „O-acyl isodipeptide unit“ Boc-Ser/ Thr(Fmoc-Xaa)-OH. Org. Biomol. Chem. 5, 1720-1730 (2007)38A. Taniguchi et al. „O-Acyl isopeptide method“ for peptide synthesis: solvent effects in the synthesis of Aβ1-42 isopeptide using „O-acyl isodipeptide unit“. J. Pept. Sci. 13, 868-874 (2007)(Fig. 5), though diketopiperazine formation is not affected by the method chosen for introducing the ester bond. It is best combated by the use of the more labile Bsmoc protecting group39I. Coin et al. Depsipeptide methodology for solid-phase peptide synthesis: circumventing side reactions40L.A. Carpino et al. The 1,1-Dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc) amino-protecting group. J. Org. Chem. 64, 4324-4338 (1999) or a less nucleophilic base. 41Yoshiya et al. O-acyl isopeptide method: efficient synthesis of isopeptide segment and application to racemization-free segment condensation. Org. Biomol. Chem. 7, 2894-2904 (2009) β-elimination during the activation step has been described by Coin et al. as a side reaction of isoacyl dipeptides 42I. Coin et al. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. 14, 299-306 (2008). To combat this. the coupling can be performed in non-polar solvents, if solubility is not an issue 43A. Taniguchi et al. „O-Acyl isopeptide method“ for peptide synthesis: solvent effects in the synthesis of Aβ1-42 isopeptide using „O-acyl isodipeptide unit“. J. Pept. Sci. 13, 868-874 (2007). Otherwise, a base-free activation procedure can be used as well. 44I. Coin et al. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. 14, 299-306 (2008)
As pseudoproline dipeptides, O-acyl dipeptides turned out to be versatile building blocks for the synthesis of difficult peptides such as β-amyloid (1-42)45Y. Sohma et al. The „O-Acyl isopeptide method“ for the synthesis of difficult sequence-containing peptides: application to the synthesis of Alzheimer‘s disease-related amyloid beta peptide (Abeta) 1-42. J. Pept. Sci. 11, 441-451 (2005), which indeed showed an improved solubility and reduced propensity for fibril formation, and insulin, where isoacyl dipeptides were inserted in both A- and B-chain46F. Liu et al. A synthetic route to human insulin using isoacyl peptides. Angew. Chem. Int. Ed. Engl. 53, 3983-3987 (2014). Peptides containing O-acyl bonds to Ser or Thr may act as soluble prodrugs, hence they have also been termed “switch peptides”47L. Saucède et al. Switch-peptides: From conformational studies to Alzheimer‘s disease. Chimia 60, 199-202 (2006) (Fig. 6).
Subscribe to our newsletter
"*" indicates required fields
References
- 1T. Haack and M. Mutter
Serine derived oxazolidines as secondary structure disrupting, solubilizing building blocks in peptide synthesis. Tetrahedron Lett. 33, 1589-1592 (1992) - 2M. Mutter et al. Pseudo-prolines (psiPro) for accessing “inaccessible” peptides. Pept. Res. 8, 145-153 (1995)
- 3T. Wöhr et al. Pseudoprolines as a solubilizing, structuredisrupting protection technique in peptide synthesis. J. Am. Chem. Soc. 118, 9218-9227 (1996)
- 4T.M. Postma and F. Albericio
Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in Fmoc-based solid-phase peptide synthesis, Org. Lett. 16, 1772-1775 (2014) - 5S. Chierici et al. A case study of 2,2 dimethylthiazolidine as locked cis proline amide bond: synthesis, NMR and molecular modeling studies of a delta-conotoxin EVIA peptide analog. Org. Biomol. Chem. 2, 2437-2441 (2004)
- 6T. Wöhr et al. Pseudoprolines as a solubilizing, structuredisrupting protection technique in peptide synthesis. J. Am. Chem. Soc. 118, 9218-9227 (1996)
- 7T.M. Postma and F. Albericio
Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in Fmoc-based solid-phase peptide synthesis Org. Lett. 16, 1772-1775 (2014) - 8T. Wöhr and M. Mutter, Pseudo-prolines in peptide synthesis:Direct insertion of serine and threonine derived oxazolidines in dipeptides. Tetrahedron Lett. 36, 3847-3848 (1995)
- 9P. White et al. Expediting the Fmoc solid phase synthesis of long peptides through the application of dimethyloxazolidine dipeptides. J. Pept. Sci. 10, 18-26 (2004)
- 10W.R. Sampson et al. The synthesis of „difficult“ peptides using 2-hydroxy-4-methoxybenzyl or pseudoproline amino acid building blocks: a comparative study. J. Pept. Sci. 5, 403-409 (1999)
- 11M. Keller and A.D. Miller Access to the inaccessible sequence of cpn 60.1 (195-217A. Abedini and D.P. Raleigh Incorporation of pseudoproline derivatives) by temporary oxazolidine protection of selected amide bonds. Bioorg. Med. Chem. Lett. 11, 857-859 (2001)
- 12A. Abedini and D.P. Raleigh, Incorporation of pseudoproline derivatives allows the facile synthesis of human IAPP, a highly amyloidogenic and aggregationprone polypeptide.
Org. Lett. 7, 693-696 (2005) - 13K. Page et al. Fast Fmoc synthesis of hAmylin1-37 with pseudoproline assisted on-resin disulfide formation.
J. Pept. Sci. 13, 833-838 (2007) - 14F. García-Martin et al. The synergy of ChemMatrix resin and pseudoproline building blocks renders RANTES, a complex aggregated chemokine. Biopolymers 84, 566-575 (2006)
- 15P. Marek et al. Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids. Org. Lett. 12, 4848-4851 (2010)
- 16S. Northfield et al. Synthesis of “Difficult” Fluorescence Quenched Substrates of Granzyme C. Int. J Pept. Res. Ther. 16, 159-165 (2010)
- 17P.W.R. Harris et al. A single pseudoproline and microwave solid phase peptide synthesis facilitates an efficient synthesis of human amylin 1-37. Int. J. Pept. Res. Ther. 19, 147-155 (2013)
- 18S. Northfield et al. Synthesis of “Difficult” Fluorescence Quenched Substrates of Granzyme C. Int. J Pept. Res. Ther. 16, 159-165 (2010)
- 19C. Sager et al. Influence of cis-trans isomerisation on pentapeptide cyclisation. Tetrahedron Lett. 40, 7987-7892 (1999)
- 20C. Sager et al. Influence of cis-trans isomerisation on pentapeptide cyclisation. Tetrahedron Lett. 40, 7987-7892 (1999)
- 21I. Coin et al. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. 14, 299-306 (2008)
- 22K. Page et al. Fast Fmoc synthesis of hAmylin1-37 with pseudoproline assisted on-resin disulfide formation. J. Pept. Sci. 13, 833-838 (2007)
- 23D. Skropeta et al. Pseudoprolines as removable turn inducers: tools for the cyclization of small peptides. J. Org. Chem. 69, 8804-8809 (2004)
- 24N. Schmiedeberg and H. Kessler Reversible backbone protection enables combinatorial solid-phase ring-closing metathesis reaction (RCM) in peptides. Org. Lett. 4, 59-62 (2002)
- 25P. Dumy et al. Pseudo-prolines as a molecular hinge: reversible induction of cis amide bonds into peptide backbones. J. Am. Chem. Soc. 119, 918-925 (1997)
- 26M. Keller et al. Pseudoproline-containing analogues of morphiceptin and endomorphin-2: evidence for a cis Tyr-Pro amide bond in the bioactive conformation. J. Med. Chem. 44, 3896-3903 (2001)
- 27T.M. Postma and F. Albericio Cysteine pseudoprolines for thiol protection and peptide macrocyclization enhancement in Fmoc-based solid-phase peptide synthesis Org. Lett. 16, 1772-1775 (2014)
- 28C. Sager et al. Influence of cis-trans isomerisation on pentapeptide cyclisation. Tetrahedron Lett. 40, 7987-7892 (1999)
- 29A. Wittelsberger et al. Introduction of a cis-prolyl mimic in position 7 of the peptide hormone oxytocin does not result in antagonistic activity. J. Med. Chem. 48, 6553-6562 (2005)
- 30Z. Qian et al. Structure-based optimization of a peptidyl inhibitor against calcineurin-nuclear factor of activated T cell (NFAT) interaction. J. Med. Chem. 57, 7792-7797 (2014)
- 31M. Pelay-Gimeno et al. Rescuing biological activity from synthetic phakellistatin 19. J. Med. Chem. 56, 9780-9788 (2013)
- 32Z. Qian et al. Structure-based optimization of a peptidyl inhibitor against calcineurin-nuclear factor of activated T cell (NFAT) interaction
- 33Y. Sohma et al. O-N intramolecular acyl migration reaction in the development of prodrugs and the synthesis of difficult sequence-containing bioactive peptides. Biopolymers 76, 344-356 (2004)
- 34S. Dos Santos et al. Switch-peptides: controlling self-assembly of amyloid beta-derived peptides in vitro by consecutive triggering of acyl migrations. J. Am. Chem. Soc. 127, 11888-11889 (2005)
- 35Y. Sohma et al. Development of O-acyl isopeptide method. Biopolymers 88, 253-262 (2007)
- 36I. Coin et al. Depsipeptide methodology for solid-phase peptide synthesis: circumventing side reactions and development of an automated technique via depsidipeptide units. J. Org. Chem. 71, 6171-6177 (2006)
- 37T. Yoshiya et al. „O-Acyl isopeptide method“ for peptide synthesis: synthesis of forty kinds of „O-acyl isodipeptide unit“ Boc-Ser/ Thr(Fmoc-Xaa)-OH. Org. Biomol. Chem. 5, 1720-1730 (2007)
- 38A. Taniguchi et al. „O-Acyl isopeptide method“ for peptide synthesis: solvent effects in the synthesis of Aβ1-42 isopeptide using „O-acyl isodipeptide unit“. J. Pept. Sci. 13, 868-874 (2007)
- 39I. Coin et al. Depsipeptide methodology for solid-phase peptide synthesis: circumventing side reactions
- 40L.A. Carpino et al. The 1,1-Dioxobenzo[b]thiophene-2-ylmethyloxycarbonyl (Bsmoc) amino-protecting group. J. Org. Chem. 64, 4324-4338 (1999)
- 41Yoshiya et al. O-acyl isopeptide method: efficient synthesis of isopeptide segment and application to racemization-free segment condensation. Org. Biomol. Chem. 7, 2894-2904 (2009)
- 42I. Coin et al. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. 14, 299-306 (2008)
- 43A. Taniguchi et al. „O-Acyl isopeptide method“ for peptide synthesis: solvent effects in the synthesis of Aβ1-42 isopeptide using „O-acyl isodipeptide unit“. J. Pept. Sci. 13, 868-874 (2007)
- 44I. Coin et al. The depsipeptide technique applied to peptide segment condensation: scope and limitations. J. Pept. Sci. 14, 299-306 (2008)
- 45Y. Sohma et al. The „O-Acyl isopeptide method“ for the synthesis of difficult sequence-containing peptides: application to the synthesis of Alzheimer‘s disease-related amyloid beta peptide (Abeta) 1-42. J. Pept. Sci. 11, 441-451 (2005)
- 46F. Liu et al. A synthetic route to human insulin using isoacyl peptides. Angew. Chem. Int. Ed. Engl. 53, 3983-3987 (2014)
- 47L. Saucède et al. Switch-peptides: From conformational studies to Alzheimer‘s disease. Chimia 60, 199-202 (2006)