VIP/PACAP Peptides offered by Bachem
Vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) belong to a family of regulatory peptides which are widely expressed in the body. Their widespread distribution is correlated with their involvement in a large variety of biological activities. Both peptides display a remarkable amino acid sequence homology.
They exert their biological effects through specific membrane receptors, belonging to the superfamily of G-protein-coupled receptors (GPCRs), named PACAP/VIP-receptors, whose signaling mechanism involves the activation of adenylate cyclase and phospholipase C cascades. Since their discovery VIP and PACAP have become the research interest of many laboratories, as reflected by the increasing number of publications related to this subject. In this brochure we present a selection of products for VIP and PACAP research.
Introduction
Vasoactive intestinal peptide (VIP) andpituitary adenylate cyclase activating polypeptide (PACAP) belong to the same family of structurally related peptide hormones that also includes secretin, glucagon, growth hormone-releasing factor (GRF), and glucagon-like peptide-1 and -2 (GLP-1, GLP- 2). It is assumed that this family of peptides resulted from the duplication of a common ancestral gene, which then diverged extensively.
Fig. 1 displays the amino acid sequences ofthe different members of the PACAP-VIPGRF-glucagon superfamily in human.
| VIP | HSDAVFTDNY10TRLRKQMAVK20KYLNSILN-NH2 |
| PACAP-38 | HSDGIFTDSY10SRYRKQMAVK20KYLAAVLGKR30YKQRVKNK-NH2 |
| PACAP-27 | HSDGIFTDSY10SRYRKQMAVK20KYLAAVL-NH2 |
| Secretin | HSDGTFTSEL10SRLRDSARLQ20RLLQGLV-NH2 |
| Glucagon | HSDGTFTSDY10SKYLDSRRAQ20DFVQWLMNT-NH2 |
| PHM | HADGVFTSDF10SKLLGQLSAK20KYLESLM-NH2 |
Fig. 1. Amino acid sequences of the different members of the PACAPVIP-GRF-glucagon superfamily in human. Residues which are underlined indicate amino acids different from those of VIP. GKR (Gly28-Lys29-Arg30) internal cleavageamidation site of PACAP-38.
Vasoactive Intestinal Peptide (VIP)
Vasoactive intestinal peptide (VIP) is a 28 amino acid peptide which was originally isolated from porcine small intestine in the early 1970s by Said and Mutt. VIP is synthesized from a peptide precursor molecule (prepro-VIP; Fig. 2), containing a VIP-related peptide called PHM (peptide with N-terminal histidine and C-terminal methionine amide) in human tissues or PHI (peptide with N-terminal histidine and Cterminal isoleucine amide), its counterpart in other mammalian species like sheep, rat or mouse.
The post-translational proteolytic cleavage of the 170 amino acid prepro-VIP by a signal peptidase in the endoplasmic reticulum (ER) yields the 148 amino acid pro-VIP.
Pro- VIP is cleaved by prohormone convertases (PCs) to VIP-GKR (prepro-VIP125-155) and PHI/PHM-GKR (prepro-VIP81-110). VIP-GKR and PHI/PHM-GKR are subsequently cleaved by carboxypeptidase B-like enzymes to VIP-G and PHI/PHM-G, which finally can be metabolized by peptidyl glycine α-amidating monooxygenase enzymes (PAM) to the biologically active C-terminally amidated VIP and PHI/PHM.
The VIP peptide precursor can also be processed by an alternative pathway, in which the dibasic cleavage site after PHI is uncleaved resulting in a Cterminally extended form, PHV (peptide with N-terminal histidine and C-terminal valine), which was found to be just as potent as VIP in relaxing smooth muscle activity.
In the endocrine system, VIP releases prolactin (PRL), luteinizing hormone (LH), and growth hormone (GH) from the pituitary. It acts on the pancreas to release either insulin or glucagon, depending on the glucose level. VIP also acts on the exocrine pancreas to increase the bicarbonate output and is present in nonneuronal tissues such as the gonads and some immune cells. During development, VIP is expressed in the embryonic brain and has trophic and mitogenic effects.
Pituitary Adenylate Cyclase Activating
Polypeptide (PACAP) Pituitary adenylate cyclase activating polypetide (PACAP) was first isolated from ovine hypothalamic tissues on the basis of its ability to stimulate adenylate cyclase activity in cultured rat anterior pituitary cells. Characterization of the peptide revealed that it comprises 38 amino acid residues (PACAP-38) and is C-terminally amidated.
As we know today PACAP occurs in two biologically active forms, PACAP-38 and an alternative form corresponding to its N-terminal 27 amino acids (PACAP-27). This is due to the fact, that the sequence of PACAP-38 contains an internal cleavageamidation site (Gly28-Lys29-Arg30), which can be used to generate this amidated 27 amino acid residues comprising form of the peptide. Analysis showed that PACAP-38 is the predominant form, but the PACAP-38/ PACAP-27 ratio varies between different tissues especially in the central nervous system, adrenal and testis.
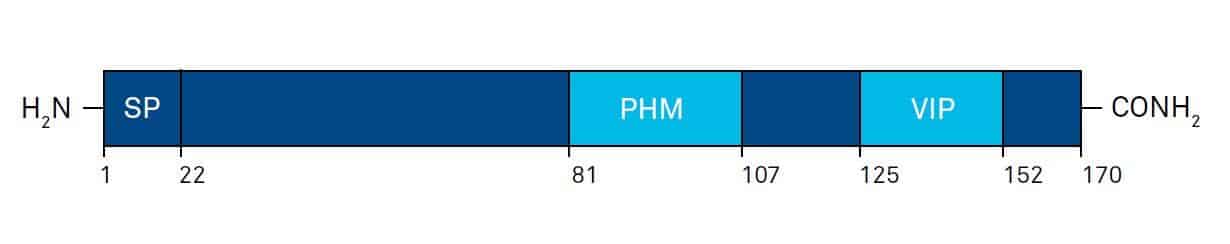
Fig. 2. Schematic illustration of the general organization of human prepro-VIP, and the location of mature PHM and VIP sequences. Amino acid numbers are shown below; SP: signal peptide. Modified after G.G. Nussdorfer and L.K. Malendowicz, Peptides 19, 1443-1467 (1998).
The N-terminal 28 amino acids of PACAP-38 show a considerable sequence homology of 68% with VIP (Fig. 1), but its adenylate cyclase stimulating activity was at least 1000 times greater than VIP. PACAP has been markedly well conserved during evolution. Its amino acid sequence is identical in rat, sheep and human, and differs in frogs and teleostean fishes by only 1 and 3 or 4 amino acid substitutions (86-92% homology), respectively.
In mammals, the overall organization of the PACAP-precursor exhibits strong similarities with the VIP precursor (Fig. 3).The posttranslational proteolytic cleavage of the 176 amino acid precursor, prepro-PACAP, results in the following peptides: PACAP-38, PACAP- 27, and a 29 amino acid PACAP-related peptide (PRP), which exhibits moderate structural homology with PACAP-27.
Like VIP, PACAP is implicated in a multitude of biological processes including reproduction, development, growth, cardiovascular, respiratory, and digestive functions, immune responses, and circadian rhythms. However, it is unclear if all these pharma pharmacological responses to PACAP are due to the physiological activities of this peptide. To answer these important questions it will be necessary to develop potent and selective PACAP antagonists and to produce PACAPand PACAP-receptor knockout animals.
Receptors
VIP and the two alternatively processed forms of PACAP elicit their biological action through binding to a subset of specific membrane receptors belonging to the large family of G-protein-coupled receptors (GPCRs). These receptors share a common molecular architecture, consisting of seven transmembrane-spanning domains (7TM), which are linked to one another by three extracellular (EC1, EC2, and EC3) and three intracellular (IC1, IC2, and IC3) oligopeptide loops, a long amino-terminal extracellular domain, and an intracellular carboxyl-terminus.
Up to now three types of receptors that can interact with PACAP and VIP have been cloned. According to the International Union of Pharmacology (IUPHAR) nomenclature these VIP/PACAP-receptors have been classified as follows: VPAC1-receptor (also known as VIP1, VIP/PACAP type II, or PVR 2) and VPAC2-receptor (also known as VIP2, VIP/PACAP type III, or PVR 3). Both receptors bind VIP and PACAP with equal affinitiy (Kd ≈ 1 nM) and activate primarily the adenylate cyclase pathway. The PAC1- receptor (also known as VIP/PACAP type I receptor, or PVR 1) shows a high affinity for PACAP-27 and PACAP-38 (Kd ≈ 0.5 nM), but a much lower affinity for VIP (Kd > 500 nM).
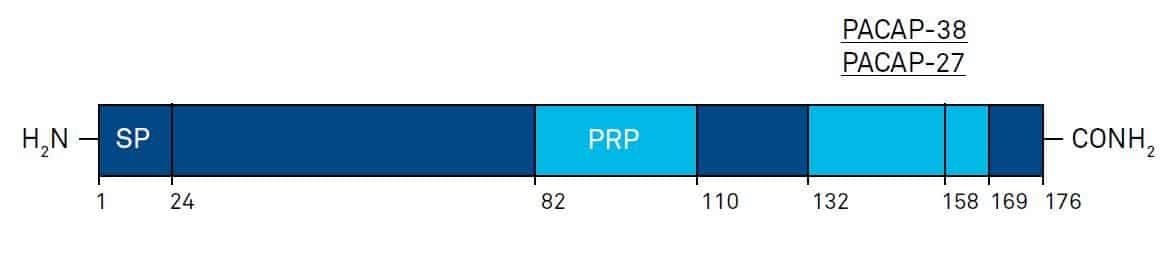
Fig. 3. Schematic illustration of the general organization of human prepro-PACAP, and the location of mature PRP; PACAP-27, and PACAP-38 sequences. Amino acid numbers are shown below; SP: signal peptide. Modifed after G.G. Nussdorfer and L.K. Malendowicz, Peptides 19, 1443-1467 (1998).
This PACAP preferring receptor activates both adenylate cyclase and phospholipase C and exists in at least 8 different variants. These variants result from alternative splicing of the transcript from a single gene and inclusion or exclusion of one or two cassettes, the hip and hop cassettes. The VPAC1-receptor is expressed in the brain, especially in the cerebral cortex and hippocampus, in the peripheral tissues such as liver, lung, and intestine, and in T lymphocytes.
The VPAC2-receptor has been localized to the thalamus and suprachiasmic nucleus, lower levels being detected in the hippocampus, brainstem, spinal cord, and dorsal root ganglia. The VPAC2-receptor has also been found to be present in a number of peripheral tissues, including pancreas, skeletal muscle, heart, kidney, adipose tissue, testis, and stomach.
Autoradiography of peripheral organs from mouse has revealed that the VPAC2-receptor is found predominantly in smooth muscle (in blood vessels and in the smooth muscle layers of the gastrointestinal and reproductive systems), the basal part of the mucosal epithelium in the colon, the lung, vasculature of the kidney, the adrenal medulla, and the retina.
The receptor is also present in thyroid follicular cells and acinar cells of the pancreas, tissues that have not been found to express the receptor in other species, and in very large amounts in the lung. The VPAC2-receptor has been associated with the binding of VIP to CD4 T cells to specifically induce an upregulation of T helper 2 type transcription factors. This upregulation consequently enhances interleukin-4 and interleukin-5 production, leading to a T helper 2 type phenotype.
PAC1 messenger RNA has been detected predominantly in the brain (olfactory bulb, thalamus, hypothalamus, dentate gyrus of the hippocampus and granule cells of the cerebellum) and in the adrenal medulla. PAC1-receptors are also expressed on the surface of enterochromaffin-like cells and in rat gastric and colonic myenteric neurons.
The wide distribution of these receptors provide clear evidence that VIP and PACAP have many target sites and functions. During the past few years, selective agonists and antagonists have been developed, which have been useful in broadening the understanding of their mechanisms of action.
Conclusions
Since their discovery many important findings on these two peptides have been published, resulting in a greater understanding of the multitude of biological functions of VIP and PACAP. Thus, VIP, PACAP and the future design of analogs – agonists and antagonists – might have a great potential in the treatment of tumors, diabetes, inflammatory bowel diseases, sepsis, rheumatoid arthritis and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease.
Subscribe to our newsletter
"*" indicates required fields
References
S.I. Said and V. Mutt
Potent peripheral and splanchnic vasodilator peptide from normal gut.
Nature 225, 863-864 (1970)
S.I. Said and V. Mutt
Isolation from porcine-intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon.
Eur. J. Biochem. 28, 199-204 (1972)
A. Miyata et al.
Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells.
Biochem. Biophys. Res. Commun. 164, 567-574 (1989)
A. Miyata et al.
Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38).
Biochem. Biophys. Res. Commun. 170, 643-648 (1990)
A. Arimura
Pituitary adenylate cyclase activating polypeptide (PACAP): discovery and currentstatus of research.
Regul. Peptides 37, 287-303 (1992)
A.J. Harmar et al.
International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide.
Pharmacol. Rev. 50, 265-270 (1998)
V. Wray et al.
Solution structure comparison of the VIP/PACAP family of peptides by NMR spectroscopy.
Ann. N.Y. Acad. Sci. 865, 37-44 (1998)
N.M. Sherwood et al.
The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily.
Endocr. Rev. 21, 619-670 (2000)
D. Vaudry et al.
Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions.
Pharmacol. Rev. 52, 269-324 (2000)
R.P. Gomariz et al.
Immunology of VIP: a review and therapeutical perspectives.
Curr. Pharm. Des. 7, 89-111 (2001)
D. Ganea et al.
Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: players in innate and adaptive immunity.
Cell. Mol. Biol. (Noisy-le-grand) 49, 127-142 (2003)
D. Pozo
VIP- and PACAP-mediated immunomodulation as prospective therapeutic tools.
Trends Mol. Med. 9, 211-217 (2003)
M. Delgado et al.
The significance of vasoactive intestinal peptide in immunomodulation.
Pharmacol Rev. 56, 249-290 (2004)
J. Fahrenkrug and J. Hannibal
Neurotransmitters co-existing with VIP or PACAP.
Peptides 25, 393-401 (2004)
I. Gozes and S. Furman
Potential clinical applications of vasoactive intestinal peptide: a selected update.
Best Pract. Res. Clin. Endocrinol. Metab. 18, 623-640 (2004)
A. Dejda et al.
Neuroprotective potential of three neuropeptides PACAP, VIP and PHI.
Pharmacol. Rep. 57, 307-320 (2005)