Corona Virus disease 2019 (COVID-19) affects us all, either directly with an infection or indirectly, through the impact other people’s infections have upon us. Severe Acute Respiratory Syndrome Coronaviruses 2 (SARS-CoV-2) enter our cells via the external part of the angiotensin-converting enzyme 2 (ACE2) receptor protein. ACE2 was found within the membranes of many cells including the nasopharynx, nasal and oral mucosa, as well as the epithelial cells of lung alveoli, small intestine, colon, kidney, liver, pancreas and vascular endothelium. ACE2 is an important component of our hormone system that regulates fluid and salt balance and thus our blood pressure. The Renin-Angiotensin-Aldosterone System (RAAS) also plays a major role for several pathological processes like the acute respiratory distress syndrome (ARDS), fibrosis, hypertension and cardiac dysfunction.
ACE2 level-influencing drugs: harmful or beneficial to COVID-19 patients?
As patients with these conditions face a more severe outcome from a COVID-19 infection, there were concerns that typical drugs for these patients which increase the ACE2 levels − like the Angiotensin-II blockers (ARBs) and Angiotensin-Converting Enzyme inhibitors (ACEi) − make such patients more susceptible to SARS-CoV-2 infections. Meanwhile, the experts are not sure anymore and they are even discussing the beneficial effects of such drugs on COVID-19 patients. This is because the viral entry process leads to a downregulation of ACE2 and ACE2 is considered to be a protective agent for inflammation, as it increases the level of the anti-inflammatory peptide Angiotensin 1-7 (Ang 1-7) and decreases the level of the pro-inflammatory vasoconstrictor peptide Angiotensin II (Ang II). To be more precise: the membrane bound receptor ACE2 converts the octapeptide Ang II directly to the anti-inflammatory Ang 1-7 and the decapeptide Angiotensin I to Angiotensin 1-9 (Ang 1-9), which can be converted subsequently by the Angiotensin-Converting Enzyme (ACE) to Ang 1-7. The peptide Ang 1-7 then binds to the mitochondrial assembly receptor (MasR) on cell surfaces to stimulate vasodilation and anti-inflammatory effects (see illustration*).
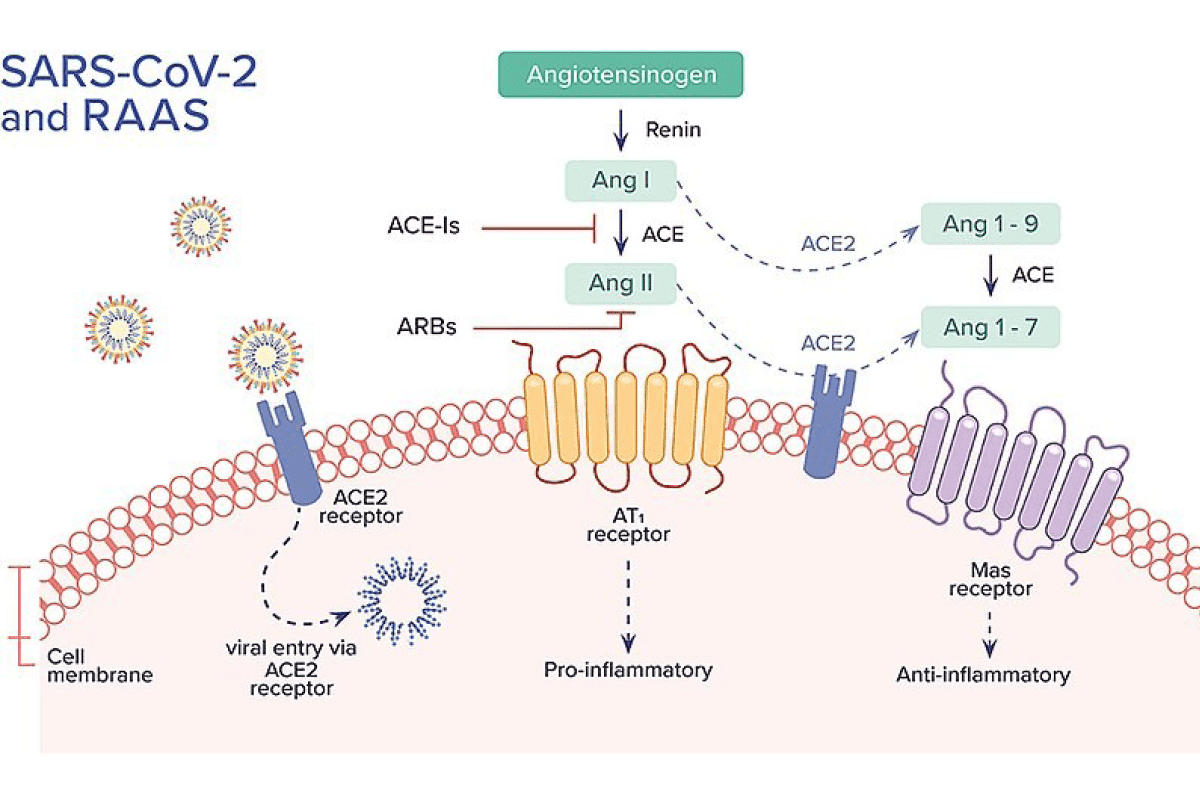
The interaction of SARS-CoV-2 with the renin-angiotensin-aldosterone system (RAAS), an important hormone system that regulates blood pressure and electrolyte balance in the body11. Chams, Nour, Sana Chams, Reina Badran, et al. “COVID-19: A Multidisciplinary Review” Frontiers in Public Health 8 (2020): 383.
In March 2020, researchers from China first reported on higher concentrations of circulating Ang II in plasma samples from COVID-19 patients using commercial ELISA tests. The higher Ang II levels in COVID-19 patients strongly correlated with the viral load and symptoms of lung injury2Liu, Yingxia, Yang Yang, Cong Zhang, et al. “Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury.” Science China Life Sciences, 63 (2020): 364–374 . They suggested that the imbalanced RAAS in COVID-19 patients was caused by SARS-CoV-2. Furthermore, they argued that drugs balancing the RAAS, such as ARBs and ACEi might be beneficial to these patients. Several studies followed showing harmful, beneficial or no effects on the severity of COVID-19 in patients treated with RAAS inhibitors. Then researchers investigated the continuation vs. the discontinuation of treatment with ACEis and ARBs in COVID-19 patients with hypertension and concluded that their use should be continued. Therefore, American and European medical societies currently recommend physicians do not change the usual ARBs and ACEi therapies in COVID-19 patients [1, 4]. However, the research community agrees that further research is needed and this, in turn, requires an accurate assessment of the angiotensins.
Assessing the angiotensins using Bachem’s research grade peptides as controls
The interaction of SARS-CoV-2 with ACE2 is important due to the therapy decisions that might follow the measurement of the angiotensins in COVID-19 patients. For this reason, US scientists did the following: They compared the results obtained with commercial ELISA tests with results obtained with a validated radioimmunoassay using the Ang 1-7 and Ang II standards from Bachem Torrance, CA3Chappel, Mark C., Nancy T. Pirro, Andrew M. South, TanYa M. Gwathmey. “Concerns on the Specificity of Commercial ELISAs for the Measurement of Angiotensin (1-7) and Angiotensin II in Human Plasma” Hypertension. 77, 3 (2021): e29-e31. The quantification of Ang 1-7 and Ang II is challenging, because the levels of these peptides in our body are in measured in picograms per milliliter plasma or per gram tissue. That’s why radioimmunoassays − given their sensitivity and relative specificity − are the standard for measuring angiotensins since 40 years. The results of the investigations of the scientists from the Hypertension and Vascular Research Center at the Wake Forest University School of Medicine mentioned above, published in January 2021, are as follows: “As to the inability of these ELISAs to recognize the Bachem Ang (1–7) and Ang II standards, either the sensitivities of the ELISAs are far lower (worse) than stated by the manufacturer or these assays do not detect authentic Ang (1–7) and Ang II. This may also explain the failure of both ELISAs to detect Ang (1–7) and Ang II in the extracted plasma samples. Nonetheless, we cannot recommend that these assays provide an accurate assessment of Ang (1–7) or Ang II in direct or extracted plasma samples.” Their advice? In a nutshell: assess plasma rather than serum samples and validate reference standards even for commercial clinical testing kits.
Inhibiting the SARS-CoV-2-ACE 2 interaction
One possible therapeutic approach for COVID-19 infection would be the blocking of the SARS-CoV-2-ACE2 interaction either with antibodies and/or some other small peptide molecules4Kumar Shuklar, Ashwin, and Monisha Banerjee. “Angiotensin-Converting-Enzyme 2 and Renin-Angiotensin System Inhibitors in COVID-19: An Update.” High Blood Pressure & Cardiovascular Prevention 28, 2 (2021): 129-139. Scientists at the University of Cambridge, UK, recently used beating stem cell-derived heart cells infected with a SARS-CoV-2 spike-pseudotyped virus system to screen potential inhibitors of a SARS-CoV-2 infection. Amongst the test compounds there was the ACE2 peptide antagonist DX600, that is − as the angiotensin peptides mentioned above − also a product of Bachem’s catalog in the application field cardiovascular system and diseases.
The Cambridge researchers demonstrated that within this screening approach DX600 could “prevent SARS-CoV-2 from entering the heart cells.” Furthermore, the synthetic peptide was even seven times more effective than the antibody against ACE2 that was also investigated. As they state in their article, “DX600 is a highly selective peptide that has not been tested previously as a viral entry inhibitor. It forms multiple interactions with the catalytic site of the ACE2 receptor that is distinct from the receptor-binding domain of the virus.”5Williams, Thomas L., Maria T. Colzani, Robyn G. C. Macrae, et al. “Human embryonic stem cell-derived cardiomyocyte platform screen inhibitors of SARS-CoV-2 infection.” Communications Biology 4, 926 (2021) Their conclusion is that binding of DX600 may be sufficient to change the conformation of ACE2 in such a way that the virus cannot interact with the receptor and enter the cells anymore. There is hope now that these promising results in cells will turn into an effective treatment for COVID-19 patients − and thus help us all.
*Illustration taken with friendly permission from https://commons.wikimedia.org/wiki/File:Fpubh-08-00383-g004.jpg
About Bachem
Bachem is a leading, innovation-driven company specializing in the development and manufacture of peptides and oligonucleotides. The company, which has over 50 years of experience and expertise, provides products for research, clinical development, and commercial application to pharmaceutical and biotechnology companies worldwide and offers a comprehensive range of services. Bachem operates internationally with headquarters in Switzerland and locations in Europe, the US and Asia. The company is listed on the SIX Swiss Exchange. For further information, see the Bachem website.
Subscribe to our newsletter
"*" indicates required fields
- 1
- 2
- 3
- 4
- 5