A peptide is a chain of amino acids, which are used as building blocks. More commonly, peptides are short proteins with varying lengths from 2 amino acids to 100 amino acids. Peptides can be found in nature and can perform biological functions such as acting as messengers in the body. If you are interested in learning more about what a peptide is please go and read our article. Peptides are very interesting molecules for many applications from therapeutics to cosmetics and diagnostics. But how are they made?
Chemical peptide synthesis is the most used method to get these molecules because it brings a lot of advantages. First, it is simpler than biological methods especially for short peptides (up to 60 amino acids). Then the chemical method brings more flexibility in scale, the sequence, and modifications that are applied. In addition to the latter advantages, chemical synthesis enables the production of a peptide that is free of Transmissible Spongiform Encephalopathy/ Bovine Spongiform Encephalopathy (TSE/BSE).
Chemical synthesis is also a convenient method to pimp peptides to provide better biological activity. In that regard, synthetic routes open ways to increase the activity of the peptide, to optimize its stability “not better, but longer”. Chemical synthesis offers more possible modifications to minimize side effects or to obtain peptides that are not natural.
How is a peptide synthesized?
The basic reaction in peptide synthesis relies on a coupling between two amino acids. They react with each other to form the dipeptide, with the separation of water. This does not happen spontaneously, so they have to be activated for the reaction to occur. For this purpose, “coupling reagents” have been developed. They generate more reactive derivatives of the amino acids and remove the water which is formed concomitantly from the system.
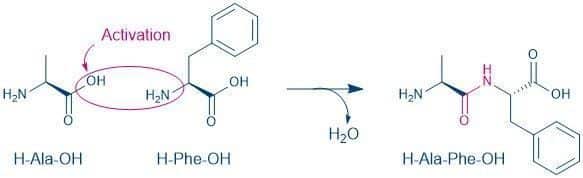
The following is an example of the synthesis of a dipeptide from two different amino acids:
L-Alanyl-L-phenylalanine (H-Ala-Phe-OH)
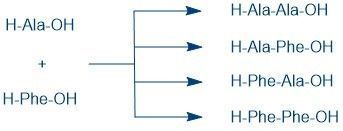
However, if a coupling reagent is simply added to a solution of alanine and phenylalanine, it results in a mixture of the four possible dipeptides; tri- and longer peptides can be produced as well:
To obtain the desired dipeptide H-Ala-Phe-OH and not a mixture, two protecting groups are needed. They must not be split off under the conditions of the coupling reaction, but be readily cleaved in a separate step after coupling has taken place.
The amino group of alanine must be blocked:
H-Ala-OH → X-Ala-OH
and the acid group of phenylalanine:
H-Phe-OH → H-Phe-OY
Now only the acid group of alanine can react with the amino group of the phenylalanine:
X-Ala-OH + H-Phe-OY → only X-Ala-Phe-OY
Finally, the protecting groups are removed:
X-Ala-Phe-OY → H-Ala-Phe-OH
i.e. X and Y are removed under the same conditions.
Now X and Y have to be selected so that X is removed under conditions in which OY is retained. In this way, longer peptides can be synthesized, which can be stretched to any length as shown in the example below.
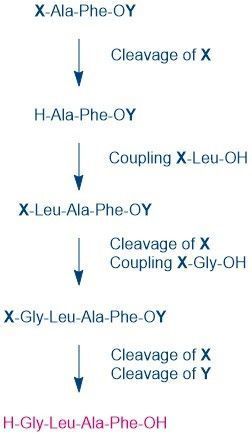
X is called a temporary protecting group because it is only used for the coupling step. Y is a permanent protecting group. It must be stable enough to endure all the coupling and X-cleavage steps. However, it must be removable in the final step, without damaging the peptide. A peptide always has two different groups at each end, and thereby a “direction”. The amino group at the end of a peptide is known as the N-terminus, the carboxyl group at the other end, the C-terminus. In chemical synthesis, the peptide is constructed from the C-terminus in the direction of the N-terminus, and therefore the terminal carboxyl group must be protected throughout the entire synthesis. Irrespective of the method of notation, when representing a peptide sequence, one starts at the N-terminus.
Protecting groups are essential to ensure the direction of the amino acid sequence
At Bachem, the main protecting groups used for the α-amine (Nα) of amino acids are Fmoc (9-Fluorenylmethoxycarbonly), Boc (t-Butoxycarbonyl), and Z (Benzyloxycarbonyl). Each of them will define the overall strategy of peptide synthesis and therefore, the deprotection and cleavage conditions will change accordingly. Fmoc will be deprotected in basic condition with a piperidine washing, whereas Boc will require acidic condition like a trifluoroacetic washing. Z will be deprotected by catalytic hydrogenation on palladium.
Many Boc or Z-amino acid derivatives are available as DCHA or CHA salts ((Di)cyclohexyl-ammonium salts). Salt formation improves the stability of the storage and handling of acid-sensitive derivatives. Indeed, solid products are much easier to handle than oils, e.g. when weighing.
Benzyloxycarbonyl (Z or Cbz) is the oldest usable Nα-protecting group for amino acids 1(M. Bergmann & L. Zervas 1932 ). The development of Z brought about the start of modern peptide synthesis and the abbreviation Z in honor of Leonidas Zervas. The Z group is often still used today to protect the amino group, also in organic synthesis.
In contrast to the α-amino group, the terminal acid group must be protected throughout the entire synthesis. The most commonly used protecting groups and their deprotection conditions are shown in the table below:
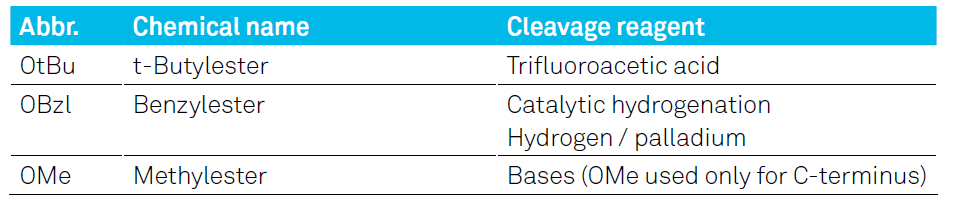
Table 1: Common permanent acid protecting group for the C-terminus
In addition to N and C-terminus of the amino acids, the side chain should be protected to avoid any side-reaction on this position during the coupling reaction. Indeed, if there is a reactive group on the side chain, for instance, the amino acid Lysine, which can react during the coupling step, this can lead to the formation of undesired ramifications and by-products. If you are interested in learning more about side-chain protection, please read our brochure.
The choice of amino and carboxyl protecting groups depends on the method and the strategy of synthesis. The two most important methods are described in the next section.
SPPS or liquid-phase? Different methods but complementary
There are two standard methods of peptide synthesis: solid-phase peptide synthesis (SPPS) and solution (or solution-phase) synthesis. The table below summarizes the similarities and differences between the two methods.
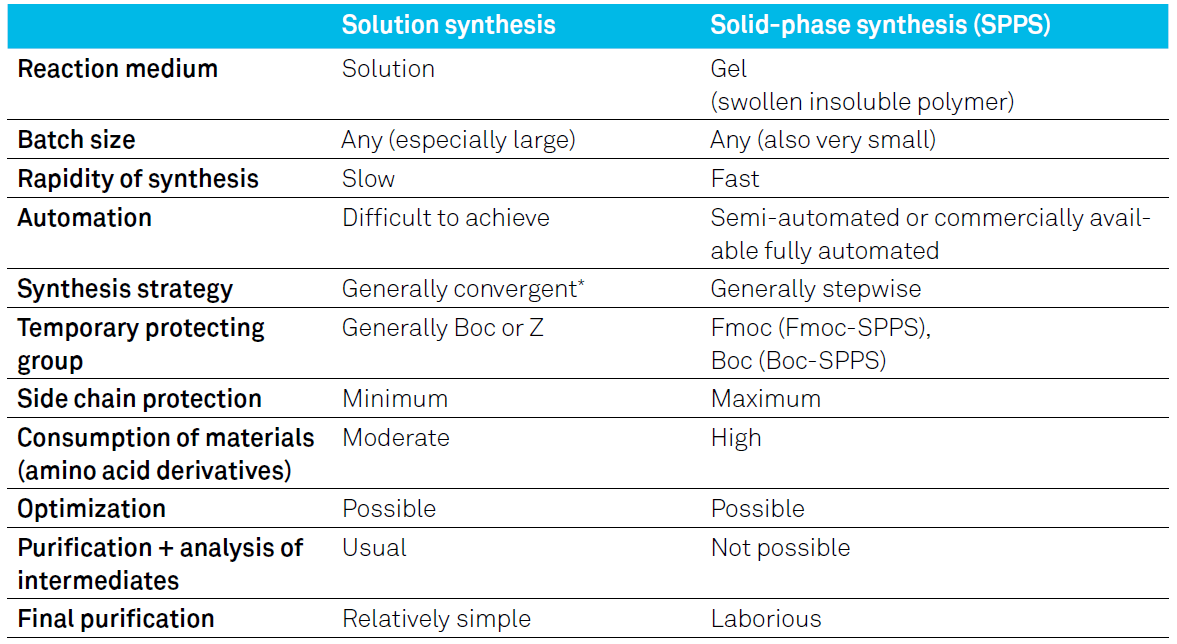
Table 2: Comparison of SPPS versus solution-phase peptide synthesis
SPPS is the core expertise of Bachem for peptides manufacturing for more than 50 years. Most peptides sold by Bachem or manufactured as contract synthesis, are produced by SPPS. Solution synthesis is still chosen for the synthesis of some of our peptide generics, for very short peptides (e.g. dipeptides), and peptides modified at the C-terminus. The essential advantages of the solid-phase synthesis over the solution synthesis are its rapidity and ease of automation.
However, it is often said that the larger the batch, the slower the SPPS proceeds, because the “manual work” increases. Small and medium quantities of peptides can be manufactured in a fully automated machine as depicted in the picture below.
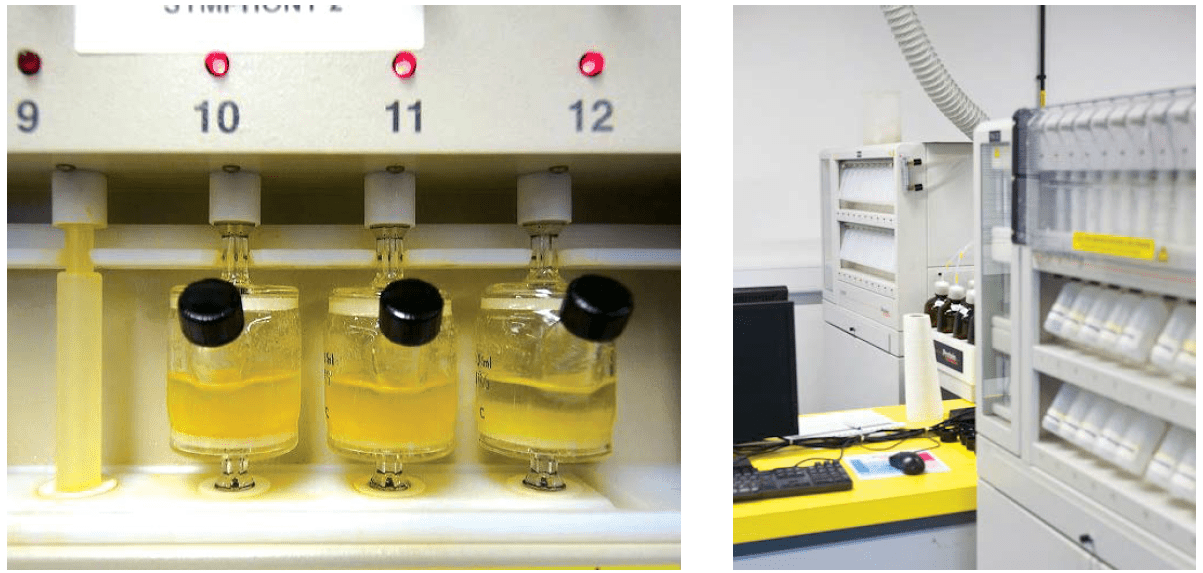
Picture 1: Peptide synthesizer, fully automated machine for SPPS. The left picture shows reactors in which synthesis is already proceeding
Very large quantities (several kg of raw peptide) are produced in semi-automated machines (only the washing program still runs automatically) or manually. Automation is possible because the peptide is constructed in steps from the C-terminus to the N-terminus. One amino acid is coupled with another.
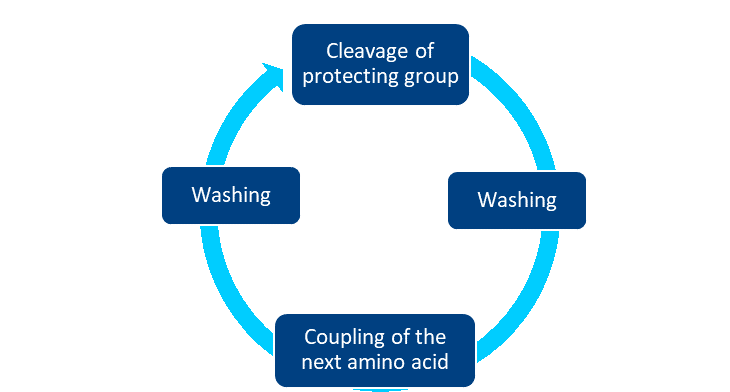
The process always includes four repetitve steps:
A solution synthesis requires careful planning. There is a standard protocol for SPPS that works for the synthesis of most peptides (although the quality of the raw peptide can be poor), but no such protocol exists for solution synthesis. There is a far greater variety with solution synthesis – not only for the possible, protecting groups – but also of coupling methods, the solvents used, and the preparative methods. Not every conceivable protecting group combination works.
Bachem is a leading, innovation-driven company specializing in the development and manufacture of peptides and oligonucleotides. For five decades, we have been innovating in our field. We are experts in solid-phase synthesis as well as cleavage and deprotection chemistry. With our strong downstream process and analytical capabilities, we provide products from development to large-scale GMP and commercialization. We strive for technology, cost leadership, business excellence, and the highest quality of our products and services for our customers.
Interested to learn more about Bachem’s world of peptides? Check out the brochure in our knowledge center!
Custom peptide synthesis
If you are interested in custom peptide synthesis for diagnostics, cosmetics or research
Subscribe to our newsletter
"*" indicates required fields
- 1(M. Bergmann & L. Zervas 1932 )