The therapeutic usage of oligonucleotide-based drugs is growing. Moving from rare diseases to chronic indications, the market demand for oligonucleotide therapeutics keeps increasing and is coupled by the emergence of new challenges related to scalability, sustainability, and cost. Recognizing this, Bachem invests in new manufacturing capacities and technologies to meet the needs of our customers. We see innovation and operational excellence as critical drivers to address the increasing demand from the market as well as from the regulatory authorities that are more and more stringent.
Over the past 50 years, we have built strong knowledge and expertise to support our customers in their Chemistry, Manufacturing, and Control (CMC) developments. To keep this leadership for our customers, we set high industry standards and our team of experts continuously improve our methods and processes. From peptides to oligonucleotides, we successfully transfer and adapt our know-how in developing and validating test methods, especially in mass spectrometry (MS) methods. From this our customers benefit from unique and innovative characterization methods for their oligonucleotide API for a successful CMC development. With our GMP-qualified equipment supporting the newest requirements, we are already developing and manufacturing oligonucleotide-based APIs together with our customers.
If you want to learn more about our QC expertise for oligonucleotides, watch our webinar!
Experts in MS analysis to support CMC development of oligonucleotides
As a new therapeutic modality, oligonucleotides present a number of challenges on regulatory aspects for the agencies and the pharmaceutical companies. With more than 15 years of MS expertise and know-how investigating peptides and oligonucleotides API, we have six high-resolution mass spectrometers in-house. With these machines, our customers benefit from precise information that supports their CMC development work. Using our knowledge in peptides, our team of experts developed easy and handy methods to characterize complex oligonucleotides and enabled a robust and specific sequence confirmation method recognizing chemical modifications.
Because of their poor pharmacokinetic and pharmacodynamic properties in the body, it has been essential to add chemical modifications to the different moieties that compose an oligonucleotide: the nucleobase, the sugar, and the backbone (Figure 1). Over the years, these molecules became more and more complex and highly modified. Furthermore, the modifications add further challenges in the separation of impurities as well as in the development of analytical methods for characterization and quantification.
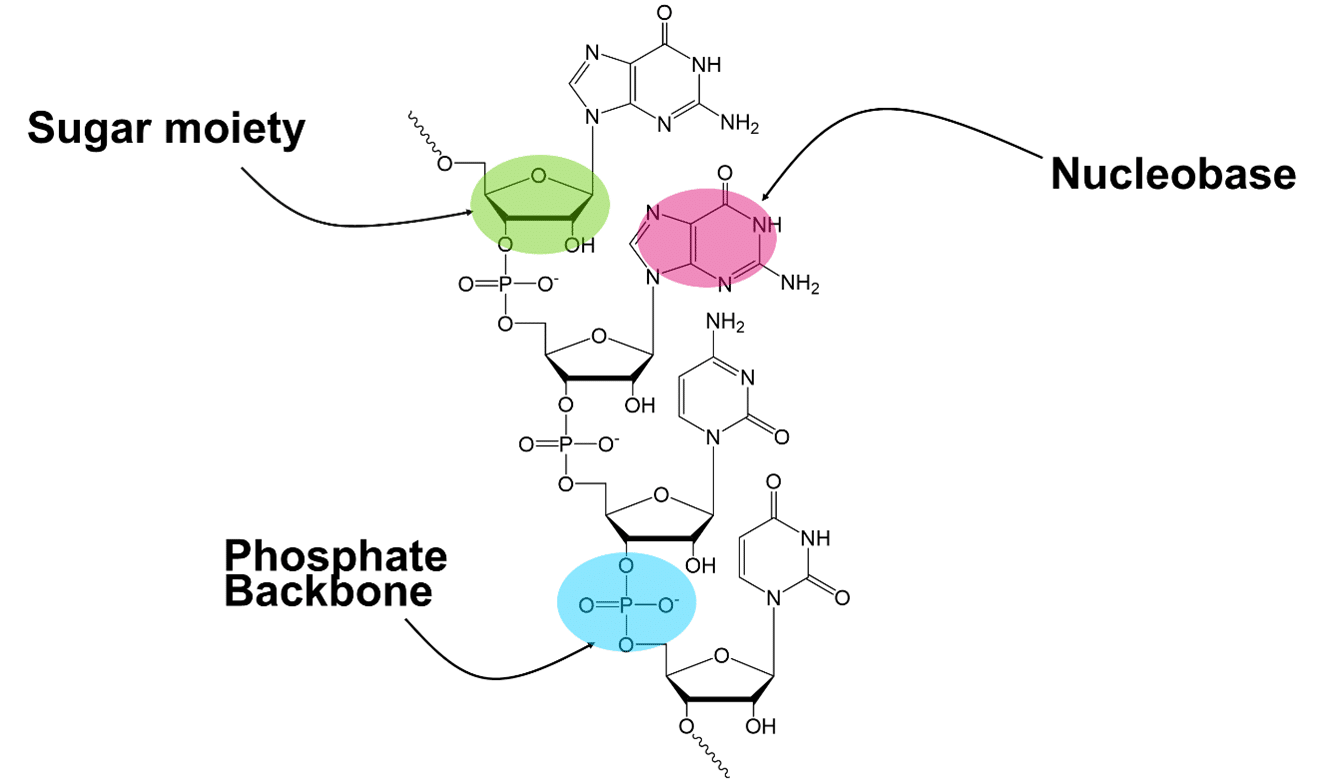
FIGURE 1 – Drawing of an oligonucleotide showing the different position of chemical modifications
With their growing complexity, there is a lack of analytical methods to accurately characterize the oligonucleotide APIs and to resolve the related impurities identification. High-resolution MS techniques present a great opportunity to overcome these challenges in comparison to well established methods such as PCR (Polymerase Chain Reaction). With a strong expertise in MS for the precise analysis of peptide API, we have seen an opportunity to develop similar methods for oligonucleotides. As for peptides, we are convinced that high-resolution MS is the most efficient method to analyze and identify modified or non-modified oligonucleotides.
Mass spectrometry (MS) in a nutshell
Mass spectrometry (MS) is an analytical technique to measure the mass of a molecule or molecular samples based on the mass-to-charge of ions. The results are represented as a mass spectrum. MS is broadly used to elucidate the chemical structure of a molecule. With our powerful equipment and software, we are able to characterize complex oligonucleotides and collect the accurate mass of the molecule.
Originally coming from the peptide world, ultra-high-resolution MS has been implemented for oligonucleotide analysis and characterization. However, the methods needed to be adapted because of the complexity of oligonucleotides. The first challenge was to develop a powerful deconvolution tool to make these complex spectra much easier and quicker to interpret. This has been achieved by the collaboration with Bruker to shift from a spectrum that is messy and difficult to interpret to a clear and straightforward result, as shown in Figure 2.
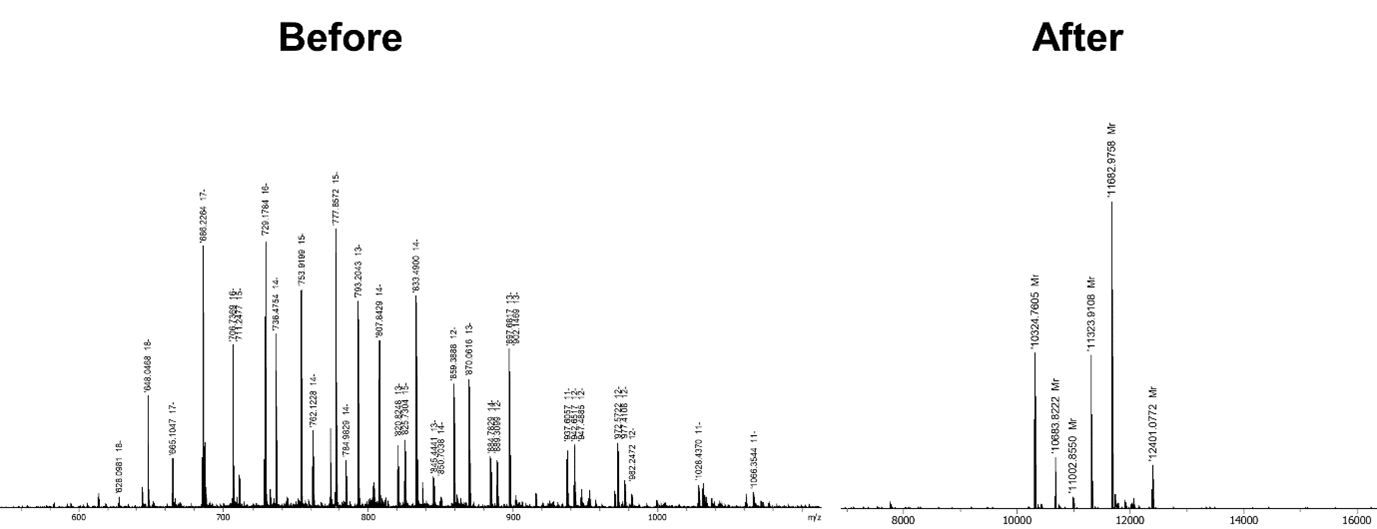
Figure 2 – Comparison before-after using the new deconvolution tool
The additional benefit to our deconvolution tool is the possibility to make the identification and quantification of impurities. This is a very handy and easy method to use with a lot of potential for automation!
Supporting the CMC development of oligonucleotides
Ultra-high-resolution MS is also a very convenient tool in combination of chromatographic techniques. Our team of MS experts can support HPLC development with a peak purity analysis and LC-MS/MS. This enables the identification and quantification of impurities that coelute with the target molecule. When producing an API, impurities are often a big challenge for manufacturers. Regulatory authorities set strict requirements on the impurities level and their identification. Therefore, having a strong and reliable process to identify the impurity profile is critical in the CMC development. With the peak purity approach, it has been possible to identify impurities in oligonucleotide APIs. Then the LC-MS/MS method has been particularly helpful for the identification of backbone sulfur (so-called PS) substitution by an oxygen, PO. This side-reaction, the PS/PO substitution, is one of the most common in oligonucleotide synthesis and is almost inevitable. Our method enables the identification of this side-reaction as well as identifying the position within the sequence when the PS/PO substitution occurs. Of course, other side-reactions such as methylation are also precisely detected.
A unique and special method for the sequence confirmation of a final oligonucleotide API
Ultra-high-resolution MS is also a powerful and sophisticated method to confirm the sequence of an oligonucleotide. Usually performed by chromatographic techniques (LC) coupled to MS equipment, our team of experts has chosen to remove the LC part due to the drawbacks and complexity it presents for GMP validation. On the other hand, MS methods are easier and faster to validate. Therefore, using ultra-high-resolution MS is advantageous in terms of time and practicability. However, some challenges have been encountered because of the specific features of oligonucleotides. Compared to peptides, the signal intensity of MS is lower because of the size of the oligonucleotides.
To gain accuracy and precision in the analysis, a strategy has been to develop an electrospray ionization MS/MS (ESI MS/MS) method using an offline desalting technique and then to directly infuse the oligonucleotide into the ESI source. This method is unique and innovative in the industry for oligonucleotide characterization and bring numerous of advantages.
The desalting and direct infusion MS is an easy and fast technique for identification of oligonucleotides. It enables an efficient MS/MS development with robust methods for sequence confirmation. It has been possible to cover the full length of both strand in a siRNA duplex and so to confirm the sequences. The analysis has also been successfully performed as a release analytics of a final API under GMP condition.
Bachem set high industry standards with innovation
At Bachem, we have a long-standing expertise in developing and validating test methods for large and structurally complicated molecules. To support our customers in their oligonucleotide development, we offer a full range of Chemistry, Manufacturing, and Control (CMC) services, including analytical services. Our very broad analytical know-how ensures successful product development. We are aware of the challenges that our customers encounter when developing oligonucleotide APIs. To help them, we anticipate the evolution of the quality expectations and standards required by the regulatory agencies.
The successful development of a very handy and precise ultra-high-resolution MS method enables the characterization of the oligonucleotide API as well as the related impurities. In addition, we developed a unique direct infusion ESI-MS/MS for a fast, robust, and generally applicable sequence confirmation method without the use of chromatographic techniques. With this unique innovation in hand, our customers benefit from a world-class analytical method for their release analysis of their final API under GMP conditions.
With our expertise and technological leadership, we set high industry standards and offer a fast and easy analytical process to our customers for their CMC development. Innovation is one of our top priorities to provide more complex molecules with superior quality. Becoming a trailblazing CDMO in oligonucleotide manufacturing will give access to innovative technologies for our customers. Ultimately, this will simplify their lives and transform the lives of patients.
About Bachem
Bachem is a leading, innovation-driven company specializing in the development and manufacture of peptides and oligonucleotides.
With over 50 years of experience and expertise Bachem provides products for research, clinical development and commercial application to pharmaceutical and biotechnology companies worldwide and offers a comprehensive range of services.
Bachem operates internationally with headquarters in Switzerland and locations in Europe, the US and Asia. The company is listed on the SIX Swiss Exchange.
Subscribe to our general newsletter
"*" indicates required fields
