MEET US AT ASIATIDES

AsiaTIDES is the premier event in Asia for accelerating promising oligonucleotide and peptide molecules from research to commercialization. The 2020 edition will take place on February 24 – 26 at the Westin Miyako Kyoto, Kyoto, Japan.
AsiaTIDES brings 300+ global oligonucleotide and peptide leaders across Asia, Europe and North America together to present case studies, best practices and to discuss current strategies and trends to accelerate promising molecules to market.
Join our presentation at the Main Conference, on Tuesday, February 25, 11:45am – 12:15pm: Dr. Ralph Schönleber, Vice President for Research & Development, Bachem AG, will present «CMC Development Concept for Synthetic Peptides».
Bachem‘s pipeline contains more than 150 customer projects in preclinical and clinical phases. In the last couple of years, a number of products in phase III trials received marketing authorization and phase II projects progressed to phase III clinical trials. Starting 2019, Bachem is strategically diversifying its technology platform to include the manufacture of therapeutic oligonucleotides and nucleic-acid-based medicine. A full-service oligonucleotide manufacturing facility is being built at the headquarters in Bubendorf, Switzerland.
The Bachem team is excited to meet with you, learn your needs for peptides and oligonucleotides and discuss how Bachem can meet your API custom manufacturing needs. We kindly invite you to drop by our Booth 19 or to contact us to schedule a meeting in advance.
We look forward to meeting you at AsiaTIDES 2020!
THERAPEUTIC OLIGONUCLEOTIDES
Oligonucleotides for therapeutic purposes gained increased importance during the past 20 years. This was furthered by advances in biomedical research, improving drug properties like degradation stability, target binding and pharmacokinetics.
Different classes of oligonucleotide therapeutics exist, referring to their mode of interaction with the target molecules. Oligonucleotides binding to mRNA rely on a Watson-Crick base-paring mechanism. The goal is to identify sequences that are highly specific for the target RNA, but do not unintendedly bind homologous RNA. However, also aspects such as tertiary structures and molecular interactions established by RNA under physiological conditions as well as aspects of drug safety and delivery have to be taken into account.
Two principle modes of action can be discriminated, a target RNA degrading and a target RNA non-degrading. Mechanisms, which decrease the expression levels of specific proteins are referred to as gene silencing. In their degrading pathways, antisense oligomers and small interfering RNAs (siRNAs) bind to corresponding sequences of mRNA and ultimately lead to the degradation of the latter. In consequence, the expression of the coded, eventually medical relevant proteins is reduced.
In their non-degrading pathways, stretches of mRNA are occupied or a steric block of the recruitment of other factors, e.g. ribosomes, takes place, leading for example to an altered mRNA splicing pattern or to a translation arrest. A further non-degrading pathway antagonizes microRNAs, endogenous small RNAs that can regulate normal physiological processes and which are involved in diseases.
Oligonucleotide aptamers do not rely on Watson-Crick base-paring, but bind via their three-dimensional structure to, typically, peptides, and consist of short strands of DNA or RNA. An example is Pegaptanib, an RNA aptamer, which received in December 2004 first approval by the US FDA for the treatment of ocular vascular disease.
Other therapeutic approaches include unmethylated CpG oligodeoxynucleotides, interesting in the context of new adjuvants, or, vaccinations employing mRNA, the latter for example investigated in the search for novel tumor therapies
Overall, therapeutic oligomers provide new chances for curing a variety of diseases, primarily on basis of information about the human genome, which differentiates them from all classical therapies.
References
[1] A.A.Levin, Treating disease at the RNA Level with oligonucleotides. N. Engl. J. Med., 380(1), 57-70 (2019)
[2] W.B.Wan and P.P. Seth, The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem., 59(21), 9645-9667 (2016)
[3] C.F.Bennett and E.E. Swayze, RNA Targeting therapeutics: Molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol., 50(1), 259-293 (2010)
PROMISE FOR OLIGONUCLEOTIDES IS RISING
Oligonucleotides have been under development for several decades. Development started with antisense oligonucleotides and aptamers, followed by siRNAs. In total, the U.S. Food and Drug Administration (FDA) has approved eleven oligonucleotide drugs.
First Approvals
In 1998, Novartis Pharmaceutical’s Vitravene (fomiversen), an antisense oligonucleotide, became the first oligonucleotide to be approved by the FDA. Vitravene was developed for the local treatment of cytomegalovirus (CMV) retinitis afflicting HIV patients. It was formulated as an intravitreal injection, a procedure to place the medication directly in the eye. Ultimately, Vitravene was discontinued in 2004 because HIV treatment led to a reduction in the number of cases of CMV in HIV patients. Macugen (peaptanib sodium), an aptamer, was approved in 2004 for the treatment of wet age related macular degeneration (AMD). Macugen is also formulated as an intravitreal injection.
Recent Approvals
Over a decade after the approval of Vitravene, FDA approval came for Kynamro (mipomersen sodium) in 2013. This approval was followed by a flurry of activity that resulted in FDA approvals for eight more oligonucleotides.
| FDA Approval | Drug name | Company | Indication | Type |
|---|---|---|---|---|
| 2013 (Withdrawn) | Kynamro (mipomersen sodium) | Kastle Therapeutics LLC | Homozygous Familial Hypercholesterolemia | Antisense Oligonucleotide |
| 2016 (Marketed) | Defitelio (defibrotide sodium) | Jazz Pharmaceuticals Plc | Hepatic Veno-Occlusive Disease | Oligonucleotide, natural product |
| 2016 (Marketed) | Exondys 51 (eteplirsen) | Sarepta Therapeutics | Duchenne Muscular Dystrophy | Antisense Oligonucleotide |
| 2016 (Marketed) | Spinraza (nusinersen) | Biogen | Spinal Muscular Atrophy | Antisense Oligonucleotide |
| 2017 (Marketed) | Heplisav-B (hepatitis B vaccine, adjuvanted) | Dynavax Technologies | Hepatitis B | Cytidine phospho-guanosine (CpG) oligonucleotide as adjuvant |
| 2018 (Marketed) | Tegsedi (inotersen sodium) | Akcea Therapeutics | Familial Amyloid Neuropathies | Antisense Oligonucleotide |
| 2018 (Marketed) | Onpattro (patisiran) | Alnylam Pharmaceuticals | Familial Amyloid Neuropathies | siRNA |
| 2019 (Marketed) | Givlaari (givosiran) | Alnylam Pharmaceuticals | Acute Hepatic Porphyria | siRNA |
| 2019 (Marketed) | Vyondys 53 (golodirsen) | Sarepta Therapeutics | Duchenne Muscular Dystrophy | Antisense Oligonucleotide |
Also in 2019, Akcea Therapeutics UK’s Waylivra (volanesorsen sodium), an antisense oligonucleotide, received conditional marketing authorization from the European Commission as an adjunct to diet for patients with familial chylomicronaemia syndrome and high risk of pancreatitis.
Drugs in Development
The clinical pipeline of oligonucleotide drugs is rich with 157 drugs in Phase I to pre-registration. The therapeutic areas for the drug candidates is diverse spanning oncology, infectious disease, metabolic disorders, genetic disorders, and other areas. Two oligonucleotide drug candidates to watch are in the pre-registration phase including Atlantic Healthcare’s alicaforsen and NS Pharma’s viltolarsen.
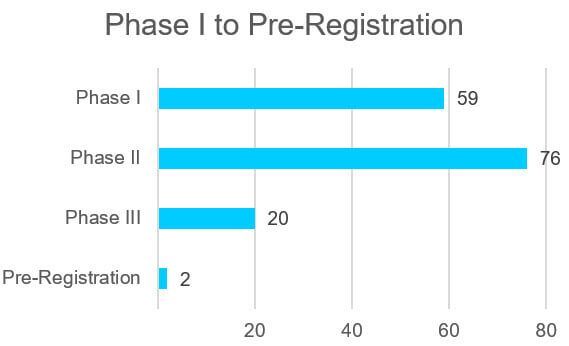
Figure 1 Oligonucleotide Clinical Drug Pipeline Phase I to Pre-Registration
References
[1] GlobalData (2019)
[2] Drugs@FDA: FDA Approved Drug Products (2019)
MEET BACHEM: ADRIEN NYAKAS
What is your official job title at Bachem?
My job title is Project Manager QC.
How long have you been with Bachem? Where did you work before Bachem?
I have been working for Bachem for 5 years, approximately 3 years in the MS-Services group and approximately 2 years as Project Manager QC. Before I did a Post Doc at the University of Bern and another one at the University of Victoria
Have you had any particular expectation when you came to Bachem and have these been fulfilled?
For the role of project manager QC I expected a profound knowledge of the aspects of analytical development of NCEs. Yes, my expectations were fully met, not only in the area of peptides but recently also in the field of oligonucleotides
Briefly, what do you do at Bachem?
I am involved in the coordination of the analytical development of peptide and oligonucleotide NCE projects.
What is your academic background/degrees or training?
I have an MSc in chemistry and a PhD in chemistry. In addition, I have a strong background in mass spectrometry.
What makes a perfect day for you?
When a big, concerted team effort results in the on-time delivery of critical data to the customer.
What do you like most about your job?
I like the fast-paced environment, close collaboration with other departments within the project teams, involvement in all aspects of a NCE project, and the direct customer contact.
What do you like to do outside of work?
I am fond of horseback riding, reading, and playing golf.
Thank you very much Adrien.

Oligo highlights
Interesting news about oligonucleotides in basic research and pharmaceutical development:
Milasen: The drug that went from idea to injection in 10 months-Chemical & Engineering News
Possible new treatment strategy against progeria-Science Daily
The discovery of RNA aptamers that selectively bind glioblastoma stem cells-Molecular Therapy Nucleic Acids
Deep genomics identifies AI-discovered candidate for Wilson Disease-Genetic Engineering & Biotechnology News
