MEET US AT TIDES VIRTUAL

TIDES is the world’s largest meeting to accelerate oligonucleotide and peptide products from early discovery to late stage development & commercialization. The 2020 edition will take place virtually on September 15 – 18, 2020.
TIDES brings 1300+ scientists and executives from 30 countries together to present case studies, best practices and to discuss current strategies and trends to accelerate promising molecules to market focused in oligonucleotide and peptide development and production.
Join our presentations:
Live presentation
Wednesday, September 16, 02:45pm – 03:15pm EST: Matteo Villain, Ph.D., Vice President, CMC, Bachem Americas, Inc., will present «An Approach to Process Validation for Peptides».
On demand presentations
FDA Draft Guidance on ANDA Filings for Peptides
Experience with the FDA Draft Guidance ANDAs for Certain Highly Purified Synthetic Peptide Drug Products That Refer to Listed Drugs of rDNA Origin
Speaker: Gerhard Haas, Ph.D., Vice President Quality Europe, Bachem AG, Switzerland
Green Chemistry
Alternative Solvents and Solvent Mixtures for Fmoc – SPPS
Speaker: Frank Dettner, Ph.D., Bachem Americas, Inc., USA
Bachem‘s pipeline contains about 150 customer projects in preclinical and clinical phases. In the last couple of years, a number of products in phase III trials received marketing authorization and phase II projects progressed to phase III clinical trials. Starting 2019, Bachem is strategically diversifying its technology platform to include the manufacture of therapeutic oligonucleotides and nucleic-acid-based medicine. A full-service oligonucleotide manufacturing facility is being built at the headquarters in Bubendorf, Switzerland.
The Bachem team is excited to meet with you, learn your needs for peptides and oligonucleotides and discuss how Bachem can meet your API custom manufacturing needs. We kindly invite you to visit our virtual Booth or to contact us to schedule a meeting in advance.
We look forward to meeting you at TIDES 2020!
SILENCING THE GENE EXPRESSION: ASOs AND siRNA THERAPEUTICS
Antisense oligonucleotides (ASOs) and small interfering RNA (siRNA) are the two most widely used strategies for silencing (specifically reducing) gene expression, with several therapies already approved or in development. ASOs and siRNA mechanistically rely on Watson-Crick base-pairing, which means they recognize specific target oligonucleotide sequences.
ASOs are synthetic, single-stranded oligonucleotides with a typical length of 16 to 21 bp. The use of oligonucleotides as therapeutic agents has elicited a great deal of interest in the last decades. However, they have poor pharmacokinetic properties. Oligonucleotides are decomposed rapidly in biological systems by nuclease cleavage and then are excreted in the urine. These limitations affect their direct use in antisense therapeutics. Therefore, it has been crucial to find modifications, which strongly improve stability of ASOs in vivo and to make ASOs druggable. Modification of the phosphate linkage has been the first successful strategy for antisense drug developments. Then modifications on the base and on the sugar moiety have been investigated to increase their binding affinity and specificity, as well as their cellular uptake (Figure 1).
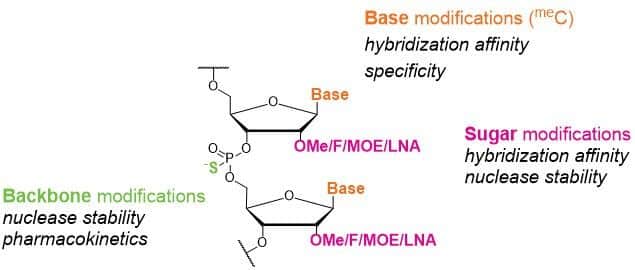
ASOs act on the DNA/RNA level and confer gene silencing in different ways: 1) formation of DNA-RNA hetero-duplexes and subsequent degradation of the target mRNA (most common mechanism, mediated by RNase H), 2) inhibition of translation by a steric blockade of the ribosome and 3) prevention of assembly of the translation machinery by interaction with the 5′-terminus of the target mRNA.
In addition to gene silencing, certain ASOs function as regulators of RNA splicing and are of therapeutic interest to restore the function or expression of a protein involved in diseases. A prominent example is the application of splice-switching ASOs for the treatment of Duchenne muscular dystrophy (DMD), overcoming a frameshift mutation and thus restoring the expression of a functional, yet truncated, dystrophin protein.
siRNAs inhibit mRNA translation, employing the RNA interference (RNAi) pathway, and have potential for the cure of virus-transmitted and genetic diseases including cancer. siRNAs initially occur as RNA duplexes, but only one strand (guide strand) induces gene silencing, while the other (passenger strand) is enzymatically degraded.
Gene silencing takes place after binding of the guide strand to a complementary mRNA sequence and subsequent cleavage of the latter, employing the RNA-induced silencing complex (RISC). Synthetic siRNA duplexes are typically 19 to 22 bp in length, which is sufficient for function, but short enough to avoid most of the interferon response triggered by duplexes larger than 30 bp in length.
While ASOs continue to be used for gene silencing, the robustness of siRNAs and the relative ease of identifying active siRNAs have increased their clinical relevance. One of the challenges remains for example delivery, since ASOs as well as siRNAs must cross biological membranes to exert their activity.
S. Karaki et al., Antisense oligonucleotides, a novel developing targeting therapy. IntechOpen, (2019)
J.K. Watts and D.R. Corey, Silencing disease genes in the laboratory and the clinic. J. Pathol., 226(2), 365-79 (2012)
A. Deiters, Small molecule modifiers of the microRNA and RNA interference pathway. AAPS J, 12(1), 51-60 (2010)
A. Khvorova and J. K. Watts, The chemical evolution of oligonucleotide therapies of clinical utility, Nat. Biotechnol., 238-248 (2017)
AFTER TWO DECADES OF RESEARCH: NEW OLIGONUCLEOTIDE THERAPIES ON THE HORIZON
Oligonucleotide therapeutics are a novel class of drug products comprised of strings of synthetic nucleotides. The potential of oligonucleotides as therapeutic drugs has generated hundreds of clinical trials. Currently, there are over 260 companies and organizations sponsoring clinical trials for oligonucleotide drug candidates.
The top five study sponsors of ongoing clinical trials of oligonucleotides include Ionis Pharmaceuticals, Alnylam Pharmaceuticals, Astra Zeneca, Biogen and Arrowhead Pharmaceuticals. Ionis is developing antisense oligonucleotides such as IONIS-AGT-LRX for the treatment of hypertension and IONIS-PKK-LRX for hereditary angioedema. Alnylam Pharmaceuticals is developing RNAi therapeutics such as lumasiran for primary hyperoxaluria type 1 and vutrisiran for the treatment of transthyretin-mediated (ATTR) amyloidosis. Meanwhile, Astra Zeneca has several oligonucleotide drug candidates in Phase I and Phase II studies such as AZD-8601 for Congestive Heart Failure and AZD-2693 for the treatment of Non-Alcoholic Steatohepatitis (NASH). In addition, Biogen has four antisense oligonucleotides currently in clinical studies for the central nervous system therapeutic area including nusinersen, tofersen sodium, BIIB078 and BIIB-094. Furthermore, Arrowhead Pharmaceuticals is leveraging RNAi technologies in several therapeutic areas including developing ARO-AAT for the treatment of liver disease associated with alpha-1 antitrypsin deficiency and JNJ-3989 for chronic hepatitis B infection.
In total, there are currently over 280 ongoing clinical trials of oligonucleotide drug candidates. The breakdown by clinical phase is shown in Figure 1.
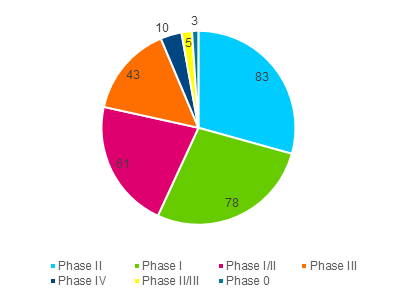
Figure 1 – Number of Ongoing Clinical Trials by Phase. For more information on clinical phase definitions, please compare for example reference 3.
The top therapeutic areas among the ongoing clinical trials is overwhelmingly oncology with 116 studies. Other top therapeutic areas include central nervous system, metabolic disorders, genetic disorders and infectious disease. The top 10 therapeutic areas for the current ongoing clinical trials are shown in Figure 2. The top indications for clinical trials of oligonucleotides include neurology, solid tumors, gastrointestinal tract cancer, skin cancer and blood cancer.
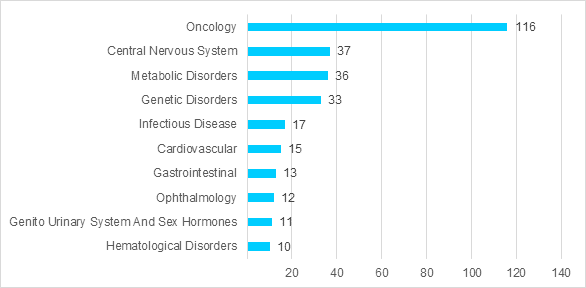
Figure 2 – Ongoing Clinical Trials by Top 10 Therapeutic Areas.
With the recent approvals in 2020 and 2019 for NS Pharma’s Viltepso®, Sarepta Therapeutics’s Vyondys 53®, Alnylam Pharmaceuticals’s Givlaari®, Akcea Therapeutics’s Waylivra® and a loaded pipeline of clinical phase drug candidates, oligonucleotide therapeutics are on the edge of delivering on the promises of two decades of research and development efforts.
References
1) Clinicaltrials.gov (2019)
2) GlobalData (2020)
MEET BACHEM: PASCAL RÖTHLISBERGER
What is your official job title at Bachem?
I am a Project Chemist.
How long have you been with Bachem? Where did you work before Bachem?
I have started in the R&D IV group at Bachem nearly four months ago. Before, I was employed as a post-doctoral fellow in a top-ranking research institute in Switzerland.
Briefly, what do you do at Bachem?
The focus of our work is set on the development and the synthesis of oligonucleotides.
What is your academic background/degrees or training?
I did my PhD in molecular life science with an emphasis on the chemistry of nucleic acids. During my post-doctoral years, I collected experience in the enzymatic synthesis of base modified oligonucleotides and the Darwinian selection of nucleic acid ligands. Additionally, I had the chance to synthesize therapeutic small interfering RNAs and evaluate their pharmaceutic potential.
What do you like to do outside of work?
I like to go on cycling tours, to play football with friends or simply go running. This helps me to get a clear mind and recharge my batteries. I also enjoy reading books and learning all sorts of new things.
What makes a perfect day for you?
On a perfect day, I will find enough time to do sports and meet with friends besides my work. Ideally, there is just little bureaucracy in order to focus on solving chemical problems. Some challenges are always nice and motivating to me.
What do you like most about your job?
I appreciate the fact, that my job here at Bachem is very versatile. I have to deal with all the facets of oligonucleotide production and I am in contact with our customers. Being a part of the oligonucleotide field, that expands so fast at the moment is fantastic. Furthermore, the great work atmosphere is contributing a lot to the quality of this job.
Have you had any particular expectation when you came to Bachem and have these been fulfilled?
After my first visit at the site in Bubendorf, I was very impressed by the oligonucleotide synthesis infrastructure that was set up within a short time. Therefore, I expected a purposeful and efficient work environment. This is exactly what I have experienced during the last months.
What do you do for fun?
Besides doing sports, I like watching football games and having a good time with my friends.
Thank you very much Pascal.

Oligo highlights
Interesting news about oligonucleotides in basic research and pharmaceutical development:
First in human study with novel antisense oligonucleotide-EurekAlert!
New muscular dystrophy treatment shows promise in pre-clinical trials-Drug Target Review
Can Biogen rebound in ALS? Maybe so, new antisense drug data suggest-Fierce Biotech
One-time treatment generates new neurons, eliminates Parkinson’s disease in Mice-UC San Diego Health
