The therapeutic usage of oligonucleotide-based drugs is growing. Moving from rare diseases to chronic indications, the market demand for oligonucleotide therapeutics keeps increasing and is coupled by the emergence of new challenges related to scalability, sustainability and cost. Recognizing this, Bachem drives innovation and invests into new oligo manufacturing capacities to meet the needs of our customers. We built facilities and implemented new, innovative solutions for large-scale oligonucleotide based Active Pharmaceutical Ingredients (API) production. With our GMP-qualified equipment, we are already developing and manufacturing oligonucleotide-based API together with our customers.
These new chemistry and manufacturing capabilities are complemented by the support of exceptional analytical chemistry teams. We have a long-standing expertise in developing and validating test methods for large and structurally complicated molecules. To support our customers in their oligonucleotide development, we offer a full range of Chemistry, Manufacturing and Control (CMC) services starting with the establishment of scalable manufacturing processes and ending with Investigational New Drug (IND) application and New Drug Application (NDA).
Innovations that solve industry challenges on API development
As a new therapeutic modality, oligonucleotides present numbers of challenges on regulatory aspects for the agencies and the pharmaceutical companies. So far, no guidelines from the International Council for Harmonization (ICH) nor from the U.S. Food and Drug Administration (FDA) have been published for the specifications of oligonucleotides quality expectations. No consensus has been found to report thresholds for impurities identification and qualification. Indeed, the nature of oligonucleotides makes them complex to characterize, as well as the impurities that can arise from their production. Most impurities exist as mixtures of closely related molecules of the target API and become tedious to separate during chromatography purification. Furthermore, there is a lack of analytical methods to accurately resolve these impurities. Therefore, having expertise in peptides is a big advantage because of the many similarities between oligonucleotides and peptides. Our world-class TIDES team is used to building in-depth know-how based on past experiences in the production of commercial API.
At Bachem, we are aware of these challenges and anticipate an evolution of the quality expectations and standards. Based on this observation, we have technologies in place to deliver improved impurity profiles accompanied with the required analytics:
Continuous chromatography for a resolved separation of product and close impurities:
Maximizing yield and quality in a sustainable, streamlined way has always been important for API production. Continuous processes are the perfect method to tackle these challenges in large-scale manufacturing. We have set up the first continuous chromatography system, a MCSGP (Multicolumn Countercurrent Solvent Gradient Purification) technology, for center-cut purification at industrial scale. The innovative MCSGP technology represents great progress in the downstream processing for peptides and oligonucleotides. The process allows a higher capacity at typically 30% lower solvent consumption, and reaches the target purity of the product often with a higher yield, typically 10 % more, compared to a conventional single-column batch purification. It is a scalable, highly efficient and cost-effective process – particularly for large scale production. It is also an automated system, which can run 24/7, allowing a significant decrease in the purification campaign cycle times. An additional benefit is that MCSGP is a more sustainable process. It reduces solvent consumption and process mass intensity (PMI). Finally, MCSGP deploys standard chromatographic conditions, and API quality is not adversely affected by changing from batch to continuous mode. This technology increases capacity, quality and sustainability of the purification process (Figure 1).
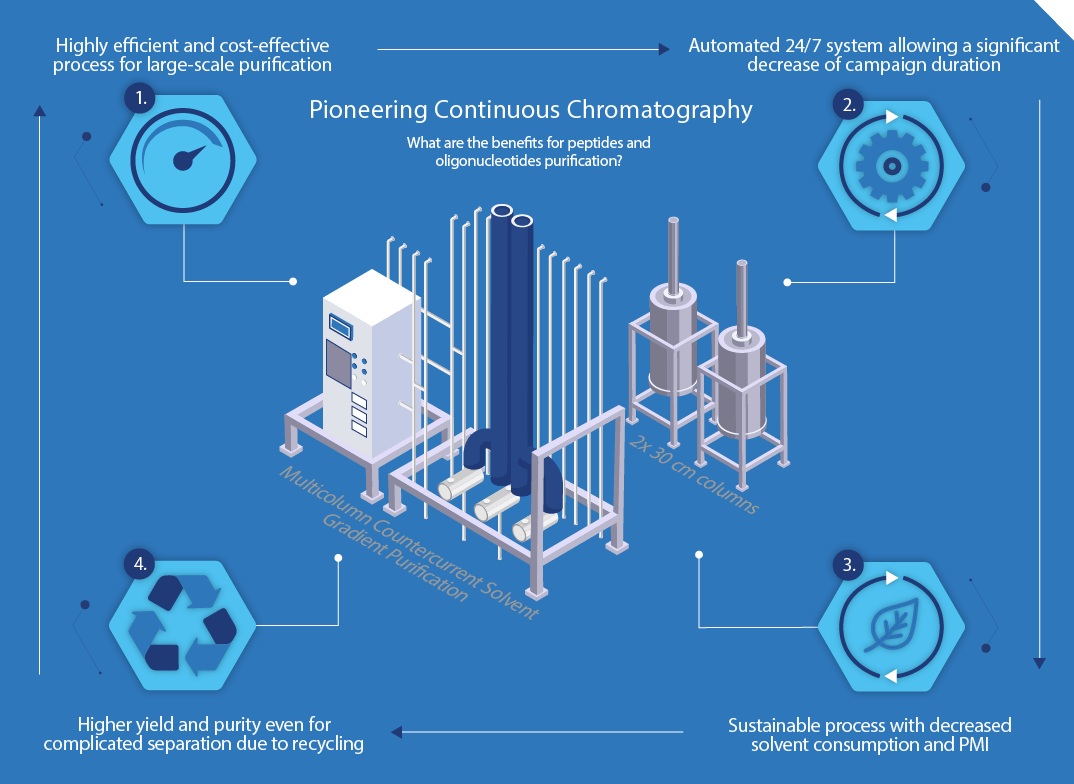
FIGURE 2 – INFOGRAPHIC SHOWING THE BENEFITS OF CONTINUOUS CHROMATOGRAPHY
New mass spectrometry (MS) characterization for an accurate product identification:
High-resolution MS is the most efficient method to analyze and identify modified or non-modified oligonucleotides. Our team of experts implemented ultra-high-resolution MS for peptides that we transferred for oligonucleotides analysis and characterization. With our powerful equipment and software, we are able to characterize complex oligonucleotides and collect the accurate mass of the molecule. Our system enables the identification and quantification of impurities. We have successfully developed a very handy and precise method that helps our customer as part of the control strategy in their CMC development. Based on our ultra-high-resolution MS, we developed a direct infusion ESI-MS/MS for a robust and specific sequence confirmation method.
Our equipment is GMP-qualified and if you want to learn more on our QC expertise for oligonucleotides, watch our presentation!
Bachem CMC offers a full package of services with risk mitigation
During a drug development, it is crucial to ensure that the product is safe, effective and of consistent quality. CMC involves these activities by defining manufacturing practices and product specifications. For our partners, we offer a clear plan for developing the CMC of peptide and oligonucleotide-based drug substances. It affords our customers a risk-mitigated approach to any clinical and commercial milestones. Our interactions with regulatory authorities and sponsors around the world lends itself to a general plan (Figure 2), which is custom-tailored to every project.
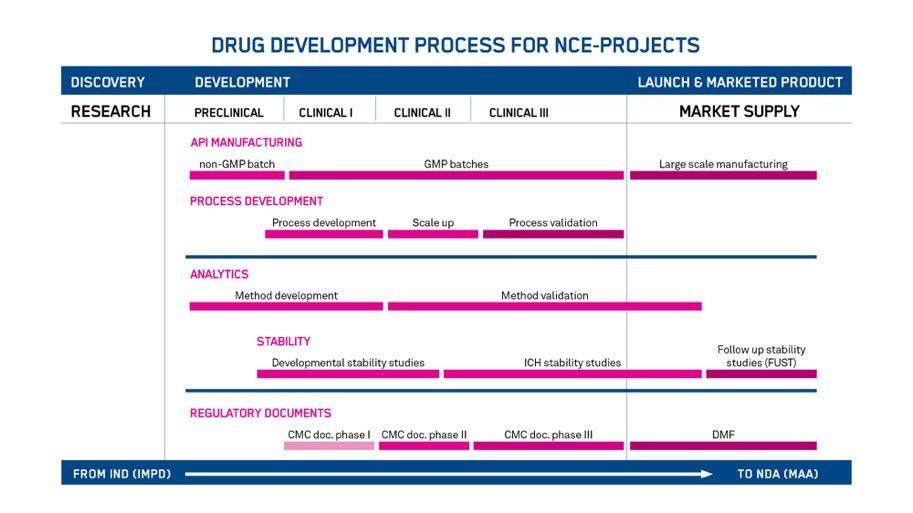
Figure 2 – Drug development process for New Chemical Entities (NCE) projects
- Method Development / Method Validation
- Stress Tests / Forced Degradation
- Stability Studies (Hold Time, Developmental, ICH, Follow UP Stability Studies (FUST))
- Impurity identification, characterization and profiling
- Characterization of aggregation / higher order structure
- Reference standard qualification
- Nitrosamine determination
- Genotoxic impurity assessment and analysis
For oligonucleotide API, we offer various support solutions for the compilation of regulatory documentation based on our international experience and according to the needs of our customers, at any stage of the product lifecycle.
Your solid partner for oligo API
Bachem has built up very broad analytical know-how and has set industry standards for peptides and now for oligonucleotides. We are confident that our background in peptides is a key asset to innovate and transform oligonucleotide manufacturing. Over several decades, we have implemented new, innovative engineering solutions to optimize the scalability, sustainability and cost-effectiveness of our peptide manufacturing to provide the highest API quality. As a proof of quality and compliance, our facilities are regularly inspected and approved by national and international regulatory authorities like FDA, Swissmedic and others. Based on our strong know-how, we now offer a full range of CMC development services for oligonucleotide-based API starting with the establishment of scalable manufacturing processes and ending with IND and NDA applications supported by in-house regulatory affairs specialists.
We are looking into the future and continue to grow with the construction of the most modern manufacturing plant for oligonucleotides. The new building will expand Bachem’s production capacities as an oligonucleotide manufacturer by 2024 and we will be ready to addresses the capacity challenges revolving around oligos targeting large patients’ populations. Innovation in this field is one of our top priorities to provide more complex molecules with superior quality and cost structure. We aim to become a trailblazing CDMO and give access to new manufacturing technologies for our partners. Ultimately, we want to simplify the lives of everyone we work with by helping our customers to develop and commercialize their API.
About Bachem
Bachem is a leading, innovation-driven company specializing in the development and manufacture of peptides and oligonucleotides.
With over 50 years of experience and expertise Bachem provides products for research, clinical development and commercial application to pharmaceutical and biotechnology companies worldwide and offers a comprehensive range of services.
Bachem operates internationally with headquarters in Switzerland and locations in Europe, the US and Asia. The company is listed on the SIX Swiss Exchange.
Subscribe to our general newsletter
"*" indicates required fields
