Peptide based drugs
They have been in the spotlight for many years due to their high therapeutic potential combining high efficacy with low toxicity. However, a peptide by itself may not be stable under physiological conditions and may therefore have a short half-life. Peptides are usually prone to proteolytic cleavage in vivo and may have to be dosed frequently to achieve the intended action. It is observed that small modifications on peptides may result in favorable changes in their drug-like properties including metabolic stability, half-life, bioavailability, toxicity etc.
Improving peptides with modifications
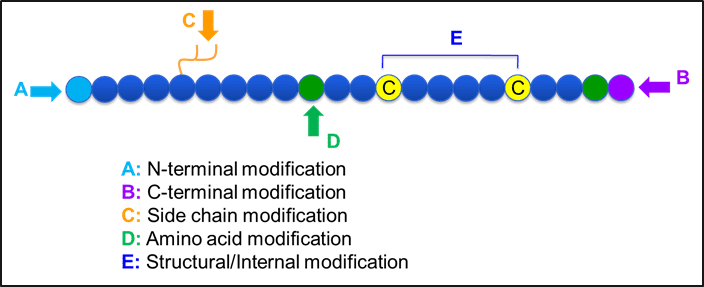
Figure-1: Frequently used peptide modifications
More common peptide modifications:
- Glycosylation
- Phosphorylation
- Cyclic peptides
- PEGylation (PEG)
- Click chemistry
- Hydrocarbon stapled peptides
- Branched peptides
- Stable isotopes labeling
- Dye labeling
- FRET labeling
- Biotinylation
Below some of the advantages in custom peptide modifications are described in more detail. They may be suitable to improve peptide drug properties. Structural alterations which may involve changes at peptide C- and N-termini, on the side chain and certain internal/backbone modifications can be used in order to study the biological function of the peptide.
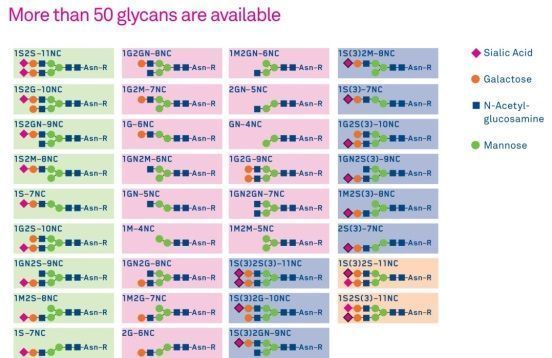
What are advantages of glycosylated peptides?
Glycosylated peptides may have improved drug properties and biological activity. Peptide glycosylation can also offer protection against proteolysis, and improve blood-brain barrier (BBB) penetration. For example, glycosylation of a linear opioid peptide amide H-Tyr-D-Thr-Gly-Phe-Leu-Ser-NH2 improved analgesia by significantly increasing its enzymatic stability in both serum and brain and by increasing its BBB permeability.
What are phosphorylated peptides?
Phosphorylation is among the most common post-translational modifications in nature, and in human cells at some point more than 30% of the proteins are phosphorylated. Phosphorylation, especially reversible phosphorylation, plays a significant role in controlling many cellular processes like signal transduction, gene expression, cell cycle and cytoskeletal regulation, and apoptosis. Deregulated phosphorylation causes various diseases, hence there is growing importance to analyze the phosphorylation state of proteins and peptides. Phosphorylation can be observed on various residues but most common phosphorylation targets are serine, threonine, and tyrosine residues.
What are cyclic peptides?
Cyclic peptides are polypeptide chains wherein the ring structure is formed via a covalent bond between amino and carboxyl terminus, amino terminus and side chain, carboxyl terminus and side chain, or side chain and side chain. Cyclic peptides have several applications in medicine as they tend to be extremely resistant to digestion and, unlike their linear counterparts, can survive in the digestive track. As they are more ‘rigid’ than the open structures they may show enhanced affinity for their target receptors.
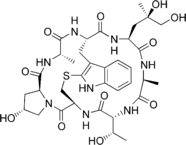
Figure-2: Phalloidin- an example for a complex cyclic peptide
They are attractive research targets and help in the engineering of peptides for the development of oral formulations.
How do PEGylated peptides work?
The short half-life of peptides, their rapid clearance (within minutes of administration) by the kidneys or the mononuclear phagocyte system and their susceptibility to degradation by proteolytic enzymes is one of the biggest challenges in the development of peptide-based drugs. Chemical modification of the peptide using polyethylene glycol (PEG) can help to overcome the above mentioned drawbacks with minimal increase in manufacturing cost. PEG is a highly investigated polymer that is used in covalent modification of biopolymers like peptides. Once linked to a peptide, each PEG subunit becomes tightly associated with two or three water molecules, which have the dual function of rendering the peptide more soluble in water and enlarging its molecular structure. As the kidneys filter substances according to size, the addition of PEG prevents the premature renal clearance undergone by small peptides. PEG’s globular structure also acts as a shield to protect the peptide from proteolytic degradation, and reduces the immunogenicity of foreign peptides by limiting their uptake through the dendritic cells.
What are click chemistry peptides?
Click chemistry is a broadly used tool to chemoselectively construct, complex peptides and is an efficient method to couple molecular fragments under mild conditions. Click chemistry reactions are highly efficient, wide in scope, stereospecific and offer an easy way to couple low- molecular fragments containing alkyne or azide functionalities. The Cu catalyzed alkyne-azide cycloaddition (CuAAC) click reaction works by “clicking” an alkyne-modified substrate with an azide-modified molecule forming a triazole link connecting the two units. Being highly chemoselective and stereospecific Click chemistry contributed to the accelerated development of peptide drugs.
What are hydrocarbon stapled peptides?
Hydrocarbon-stapled peptides are mini-proteins locked into their bioactive alpha-helical conformation through site-specific introduction of a chemical brace, an all-hydrocarbon staple. The synthesis protocol requires incorporation of α-alkenyl -α-methyl–amino acids allowing the ring-closing olefin metathesis (RCM) of the resulting resin-bound peptides.
High conformational instability of peptides leading to proteolytic cleavage and low bio-availability can be tackled via conformational stabilization of the alpha-helical structure.
For what are branched peptides used?
Branched peptides are used as antigens to produce antibodies, as toxin peptides for tumor targeting and for gene transfection. They are known to stimulate the immune system when used in the form of vaccines. They are used to act against a broad range of infectious diseases, in the form of novel drugs, vaccines or carriers for gene therapy. Amphiphilic branched peptides are also used in packaging systems for drug delivery to overcome problems associated with lipid and viral based delivery systems.
What are the benefits of stable isotope-labeled peptides?
Stable isotopes are useful in the quantification of complex proteins and peptides at very low concentrations and for monitoring the relative abundance of proteins and peptides by mass spectrometry. They are used in molecular structure studies, elucidation of metabolic pathways, protein interactions, metabolism studies, metabolic research, diagnosis and much more.
What are dye labeled peptides?
Dye-labeled peptides have been used as powerful tools to investigate protein structures and relevant biological interactions such as receptor binding. The fluorophores can be incorporated at many positions (depending upon the need) before removing the protecting groups from the amino acids. Most of the fluorescent dyes are resistant to harsh deprotection conditions. Fluorescein isothiocyanate (FITC), carboxyfluorescein (FAM), carboxytetramethylrhodamine (TAMRA, 5 or 6 isomer or mixture of isomers), 7-methoxycoumarinyl-4-acetyl, aminomethylcoumarin, 5-(dimethylamino)naphthalene-1-sulfonyl, Dansyl, Bodipy are a few to mention in the series. Dye labeled peptides have found applications in medical diagnostics, especially in the field of cancer.
What are FRET labeled peptides?
The principle of FRET (Fluorescent Resonance Energy Transfer) is commonly used with substrates for assaying protease activity and to investigate protein-protein interactions. In protein kinase assays, FRET can serve as a safe alternative to radioisotope labeling.
What are the benefits of biotinylated peptides?
Biotin has a very high binding affinity for strepatavidin and avidin which is the basic principle behind the use of biotinylated peptides in numerous biochemical applications including immunoassays, histocytochemistry, and flow cytochemistry. Biotin can be covalently linked to either the N-terminus, C-terminus or to a lysine side-chain. For peptides that interact with large molecules like antibodies or macromolecules it is recommended to insert a spacer arm between the biotin group and the amino acid.
