MEET US AT THE DCAT

Bachem is participating in the DCAT Week 2017, the pharmaceutical industry’s premier event for innovator and generic drug manufacturers and suppliers of ingredients, development and manufacturing services.
The 2017 edition will take place on March 20 – 23, 2017 in New York City, USA.
We are excited to meet with our customers at the DCAT Week and discuss how Bachem can help with their generic API manufacturing needs. With our capacity to produce generic peptide APIs in quantities of hundreds of kilograms and small molecules in tens of tons per year, our Swiss and US GMP manufacturing facilities regularly inspected by the FDA and local authorities, and our record of over 80 DMF filings in the pipeline, we will certainly be able support the success of your projects.
A new service in our portfolio is the selective chemical glycosylation. The technology is applicable to large scale and has the potential to be applied to a variety of peptides, where we can pioneer the concept of improving current and future drugs. The Bachem team at DCAT will be pleased to give you more detailed information.
We invite you to visit us at the Hotel Omni Berkshire Place, Madison Suite 1212: please contact us to schedule a meeting in advance.
We look forward to meeting you at DCAT Week 2017!
CASPASES AS REGULATORS OF APOPTOSIS
Apoptosis
Apoptosis, also called programmed cell death, is an essential physiological process of cellular autodestruction. It plays important roles in development, maintenance of cell homeostasis, and host defense in multicellular organisms [1]. Unregulated cell death is involved in a number of clinical disorders. Excessive apoptosis can lead to neurodegenerative diseases and ischemic injuries, whereas cancer and autoimmune diseases can result from insufficient apoptosis [1, 2]. Diverse cell types can be triggered to undergo apoptosis by a variety of physiological and pathological stimuli, derived from either the extracellular or intracellular milieu [2]. Cells undergoing apoptosis exhibit a series of characteristic morphological changes, including chromatin condensation, plasma membrane blebbing, cell body shrinkage and disassembly into membrane-enclosed vesicles (apoptotic bodies), which in vivo are quickly eliminated by phagocytosis [1-3]. Thus, cellular constituents are not released from dying cells, and no inflammatory response occurs [1, 2]. This is one essential feature of apoptosis, which distin-guishes it from necrosis [4].
Caspases
The central component of this machinery is a proteolytic system involving caspases (cysteine-dependent aspartate specific protease) [5]. These proteases have high specificity for substrates containing an aspartic acid residue, and use a cysteine side chain as a nucleophile during peptide bond hydrolysis [2]. Caspases are key players in apoptosis, and their activation is generally considered as the “point of no return” in apoptotic pathways [6]. To date, 18 mammalian caspases are reported, although this number is influenced by inconsistencies in the nomenclature. Examples for apoptotic caspases are caspases -2, -3, -6, -7, -8, -9 and -10. Inflammatory caspases include caspases -1, -4, -5, -11 and -12 [7]. Caspases are synthesized as latent precursors or procaspases with the general organization shown (Figure). All procaspases contain a highly homologous protease domain, which can be further divided into two subunits [1]. In some procaspases the subunits are additionally separated by a short interdomain linker. Each procaspase also contains a prodomain or NH2-terminal peptide of variable length [1].
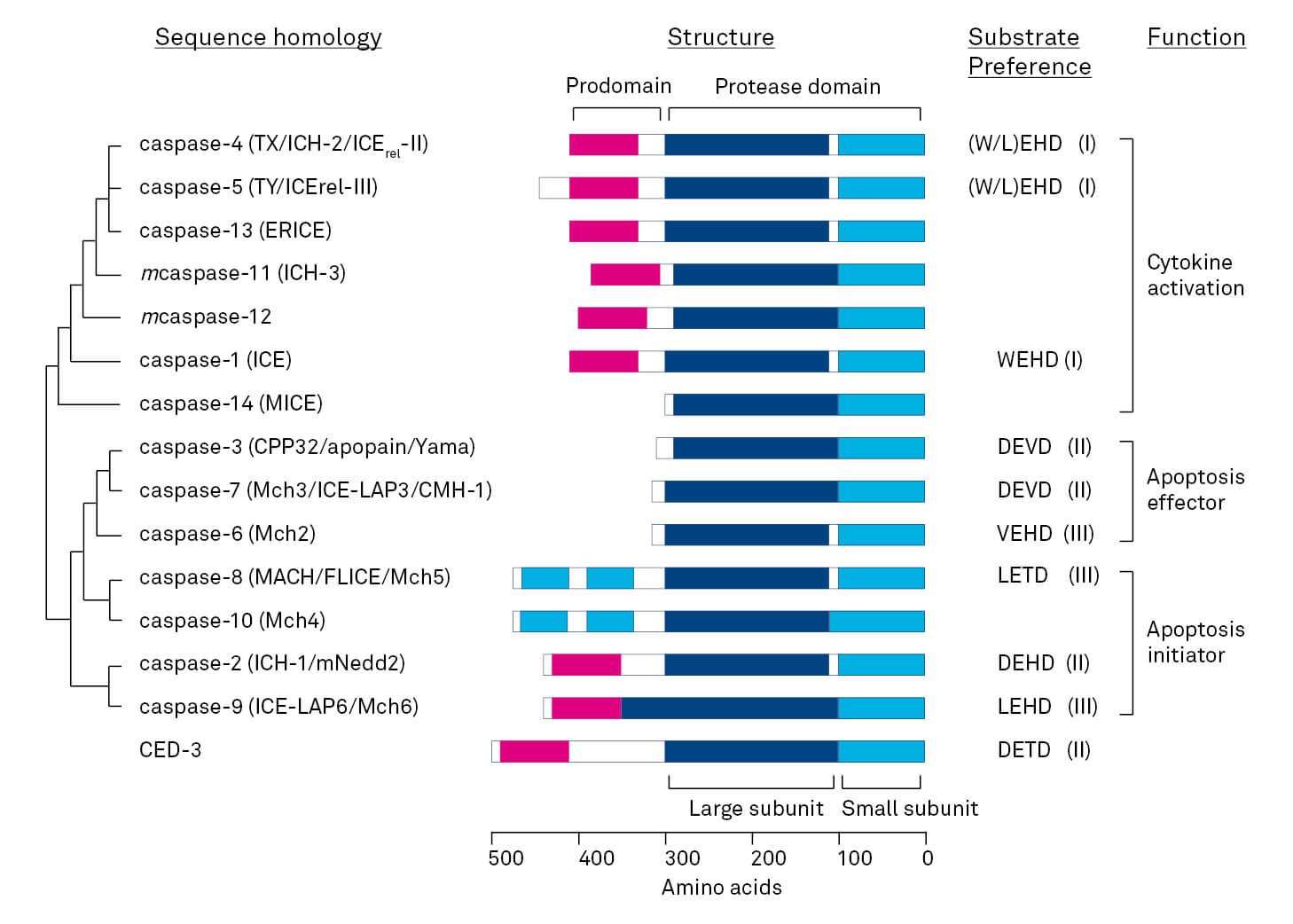
Figure: Mammalian caspase family and C. elegans caspase CED- 3 [1]. All mammalian caspases are of human origin except for murine caspase-11 and -12, for which no human counterparts have been identified yet. Substrate preferences at the P1 to P4 positions are indicated (amino acids in one-letter-code). Based on the substrate specificity, caspases are divided into three groups (indicated in parentheses). Cyan: death effector domains (DED), magenta: caspase recruitment domain (CARD).
Caspases mediating apoptosis can be discriminated into “initiator” caspases, which are activated by cellular signaling, and “effector” or “executioner” caspases, which are activated by the initiator caspases [6]. The latter takes place by proteolytic processing at aspartic acid residues and following a proteolytic cascade [1, 4]. Besides their role in apoptosis, caspases are important for a number of non-apoptotic cellular processes. The latter include non-apoptotic cell death like necroptosis, autophagy and pyroptosis, but also the activation of inflammasomes and diverse events during tissue differentiation [7, 8].
Initiation Pathways
Two major pathways are known for activation of the initiator caspases. The “intrinsic” or mitochondrial pathway is a response to cellular stresses like DNA damage or cytotoxins [6]. Regulated by the B-cell lymphoma 2 (Bcl-2) family of proteins, several factors form the “apoptosome” and recruit and activate initiator caspase-9 [9]. In the “extrinsic” or death receptor mediated pathway, receptors of the tumor necrosis factor (TNF) receptor superfamily are activated in the first step [10]. This leads to formation of the death-inducing signaling complex (DISC) and activation of specific initiator caspases, which is caspase -8 in humans and mice [6, 11]. The protein-protein interactions for formation of the DISC are established by death effector domains (DEDs), which caspases of the extrinsic pathway have in common with other DISC-forming protein components [6, 12]. In many cases, the formation of caspase oligomers, or the interaction of caspases with other proteins involves caspase recruitment domains (CARDs). Both, DEDs and CARDs comprise “death fold” motifs, which are found in other proteins as well, modulating apoptosis and inflammation [12]. Once activated the initiator caspases process and activate one or more of the effector caspases. All initiation pathways thereby seem to converge at the same execution cascade, with caspase -3 as the main effector caspase downstream [3, 13, 14]. In the following, the activated effector caspases cleave various cellular proteins, which finally leads to apoptotic cell death [15]. Since they are implicated in manifold and severe pathological disorders, factors modulating apoptosis are interesting but likewise challenging therapeutic targets [16-19]. Bachem offers a broad selection of inhibitors and research peptides, which are used to investigate the underlying principles of caspase inhibition and –activation and their impact on regulated cell death.
Explore our broad offering of caspase inhibitors and substrates and further technical information
Caspase Inhibitors and Substrates
Monograph Caspase Inhibitors and Substrates
References
[1] H.Y. Chang and X. Yang, Proteases for cell suicide: functions and regulation of caspases. Microbiology and Molecular Biology Reviews, 64(4), 821-46 (2000)
[2] M.G. Grütter, Caspases: key players in programmed cell death. Current Opinion in Structural Biology, 10(6), 649-55 (2000)
[3] B. Zhivotovsky, Caspases: the enzymes of death. Essays in Biochemistry, 3925-40 (2003)
[4] H.R. Stennicke and G.S. Salvesen, Properties of the caspases. Biochimica et Biophysica Acta, 1387(1-2), 17-31 (1998)
[5] D.W. Nicholson and N.A. Thornberry, Caspases: killer proteases. Trends in Biochemical Sciences, 22(8), 299-306 (1997)
[6] A. Degterev et al., A decade of caspases. Oncogene, 22(53), 8543-67 (2003)
[7] S. Shalini et al., Old, new and emerging functions of caspases. Cell Death and Differentiation, 22(4), 526-39 (2015)
[8] S.M. Man and T.D. Kanneganti, Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nature Reviews Immunology, 16(1), 7-21 (2016)
[9] J.M. Adams and S. Cory, life-or-death decisions by the Bcl-2 protein family. Trends in Biochemical Sciences, 26(1), 61 (2001)
[10] U. Gaur and B.B. Aggarwal, Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochemical Pharmacology, 66(8), 1403-8 (2003)
[11] E.E. Varfolomeev et al., Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity, 9(2), 267-76 (1998)
[12] J.S. Riley et al., DED or alive: assembly and regulation of the death effector domain complexes. Cell Death and Disease, 6e1866 (2015)
[13] K.M. Boatright and G.S. Salvesen, Caspase activation. Current Opinion in Cell Biology, (70), 233-42 (2003)
[14] H.R. Stennicke and G.S. Salvesen, Caspases – controlling intracellular signals by protease zymogen activation. Biochimica et Biophysica Acta, 1477(1-2), 299-306 (2000)
[15] E.A. Slee et al., Serial killers: ordering caspase activation events in apoptosis. Cell Death and Differentiation, 6(11), 1067-74 (1999)
[16] B.R. Gastman, Apoptosis and its clinical impact. Head & Neck, 23(5), 409-25 (2001)
[17] J.C. Reed, Apoptosis-regulating proteins as targets for drug discovery. Trends in Molecular Medicine, 7(7), 314-9 (2001)
[18] A. Philchenkov, Caspases: potential targets for regulating cell death. Journal of Cellular and Molecular Medicine, 8(4), 432-44 (2004)
[19] M.J. Roy et al., Cell death and the mitochondria: therapeutic targeting of the BCL-2 family-driven pathway. British Journal of Pharmacology, 171(8), 1973-87 (2014)
PEPTIDE-BASED CASPASE INHIBITORS AND ACTIVATORS IN DEVELOPMENT
Caspases are involved in apoptosis, play a role in inflammation and contribute to biological processes. An increase in apoptosis and caspase activity are associated with many different disorders such as hepatic and transplant injuries, myocardial infarction and neurodegenerative diseases. Pro-inflammatory caspases are involved in arthritis and psoriasis. Diminished caspase activity is associated with cancers (1). While there are currently no approved peptide drugs based on caspase inhibition or activation, there are several in preclinical to Phase II development. Peptide-based drug candidates known to be caspase inhibitors and activators are shown in Table 1 below.
Table 1: Peptidic Caspase Inhibitors and Activators in Development Preclinical to Pending Approval (2)
| Product Name | Active Ingredient | Condition Treated | Highest Phase | Companies | Mechanism of Action |
|---|---|---|---|---|---|
| NNZ2566 | trofinetide | Fragile X Syndrome(II), Rett's Syndrome(II), Traumatic Brain Injury(II), Others(PC) | Phase II | Neuren Pharmaceuticals Limited | Caspase-3 Inhibitor |
| VRCTC310 | cardiotoxin, crotoxin | Oncology(I) | Phase I | Spotlight Innovation Inc, Celtic Biotech Iowa Inc | Caspase-9 Activator, Secretory Phospholipase A2 (sPLA2) Inhibitor |
| AMRS001 | mesencephalic astrocyte- derived neurotrophic factor | Alzheimer's Disease(PC), Cerebral Ischemia(PC), Genetic Disorders(PC), Ischemic Heart Diseases(PC), Metabolic Disorders(PC), Optic Nerve Disorders(PC), Ototoxicity(PC), Pancreatic Diseases(PC), Parkinson's Disease(PC), Retinal Artery Occlusion(PC), Retinitis Pigmentosa(PC), Traumatic Brain Injury(PC) | Preclinical | Amarantus BioScience Holdings Inc, Generex Biotechnology Corporation | Caspase-3 Inhibitor, Gamma-Aminobutyric Acid Type A (GABAA) Receptor Agonist, Gamma-Aminobutyric Acid Type A (GABAA) Receptor Antagonist |
| Cardiotoxin | -- | Chronic Kidney Failure(PC), Oncology(PC) | Preclinical | Spotlight Innovation Inc, Celtic Biotech Iowa Inc | Caspase-9 Activator |
| CVXL0103 | -- | HER2-Negative Breast Cancer(PC), Ovarian Cancer(PC) | Preclinical | CleveXel Pharma SAS | Caspase-9 Inhibitor |
| F573 | -- | Acute Liver Failure(PC) | Preclinical | Immune Pharma-ceuticals Inc, Shanghai Genomics Inc, GNI Group Ltd | Caspase Inhibitor |
| PEP-010 | -- | Oncology(PC) | Preclinical | Inserm, CleveXel Pharma SAS, PEP-Therapy | Caspase-9 Inhibitor |
Preclinical Candidates
Amarantus BioScience is developing AMRS001, a growth factor known as mesencephalic astrocyte-derived neurotrophic factor (MANF). The lead indication for AMRS001 is retinitis pigmentosa and other indications include Parkinson’s disease, Wolfram’s syndrome, diabetes and retinal arterial occlusion (3). MANF has been granted Orphan Drug designation for the treatment of retinitis pigmentosa from both the US Food and Drug Administration and the European Medicines Agency (2). Celtic Biotech Iowa, a subsidiary of Spotlight Innovation, is developing Cardiotoxin, a membrane-disruptive peptide from Naja naja atra venom, for the treatment of chronic kidney disease (CKD) and cancer. This cobra venom peptide is a Caspase-9 activator. Positive preclinical study data has been published showing that Cardiotoxin has the ability to reduce the effects of CKD (4). CVXL0103 is a cell-penetrating peptide and caspase-9 inhibitor that induces apoptosis. This peptide was in preclinical studies for the treatment of her2-negative breast cancer and ovarian cancer by Clevexel Pharma SAS. No recent development has been reported by Clevexel Pharma and the product’s status is currently unknown (2). F573 is a dipeptide caspase inhibitor in preclinical development. GNI Group and its subsidiary, Shanghai Genomics, are developing this dipeptide for the treatment of acute liver failure. In 2011, Shanghai Genomics filed an IND for F573 in China. In 2012, GNI Group published results from a research study in mice concluding that F573 had a therapeutic effect on mice with acute liver injury (2). PEP-010 is being developed by PEP-Therapy for oncology indications. This 30-mer peptide contains DPT, a shuttle sequence that delivers the active peptide into the cell and an interfering active peptide sequence that blocks caspase-9/PP2A interaction. Preclinical studies showed that PEP-010 induced death in human tumor cell lines and in B-cells of patients with lymphocytic leukemia without affecting healthy cells. As a next step, the PEP-Therapy plans to initiate toxicity studies (5).
Phase I Candidate
VRCTC310 contains the snake venoms crotoxin and cardiotoxin. Celtic Biotech Iowa, a subsidiary of Spotlight Innovation, is developing VRCTC310 for the treatment of hematological cancers and pancreatic cancer (2). The company is preparing for Phase II studies and plans to submit an IND for VRCTC310 to the US Food and Drug Administration in 2017 (4).
Phase II Candidate
Neuren Pharmaceuticals is developing a peptide product candidate, designated NNZ-2566 (trofinetide), for Rett syndrome, Fragile X syndrome and traumatic brain injury. This candidate is a synthetic analog of glypromate, a neuropeptide derived from insulin-like growth factor-1 (IGF-1). In 2015, Neuron reported results from Phase II trials in Fragile X syndrome in which improvements were seen across the core symptoms of Fragile X syndrome. In 2016, Neuron announced that it started a Phase II trial in children and adolescents with Rett syndrome, a neurological disorder. The US Food and Drug Administration and the European Medicines Agency have both granted Orphan Drug status to trofinetide for Rett Syndrome. In addition, the FDA has granted Fast Track designation to trofinetide (2).
Conclusion
The development of therapeutics that target caspases has been of great interest and caspase inhibitors and substrates have been used to help define the role of caspases in apoptosis and other biological processes. To assist researchers and organizations that are active in this field of research, Bachem offers 85 different caspase inhibitors and substrates from stock at shop.www.bachem.com. In addition, comprehensive custom peptide synthesis services and the production of peptide-based New Chemical Entities are offered.
References
(1) J. Kudelova et al. Pharmacological Caspase Inhibitors: Research Towards Therapeutics Perspectives, J. Physiol. Pharmacol. 66, 473-482 (2015)
(2) Medtrack (2017)
(3) MANF, Amarantus Bioscience (2017)
(4) Spotlight Innovation Company Overview, Spotlight Innovation (2016)
(5) Products, PEP-Therapy (2017)
MEET BACHEM: SANDRA SCHMIDHOFER
What is your official job title at Bachem?
Project Manager
How long have you been with Bachem? Where did you work before Bachem?
I joined the project management team at Bachem in August 2015.
Briefly, what do you do at Bachem?
At Bachem I’m leading several customer-related development project teams from first ideas to market approval and beyond. It is my responsibility to ensure the progress of the project. Together with our interdisciplinary teams we seek solutions to complex issues in order to create value for our customers.
What is your academic background/degrees or training?
I studied Biology in Regensburg and did my PhD in Molecular Biology/ Epigenetics.
What do you like to do outside of work (interests, hobbies)?
In my spare time I like to do Yoga, go skiing and play Volleyball. I also love to read, good food and travelling the world.
How big is the PM team at Bachem and how are they set up?
We are a young team of 13 project leaders. PM is integrated into the Business Development & Sales department and located in the TIBA area at Bachem Bubendorf.
What makes a perfect day for you?
A perfect day includes sunshine, a good book and a cup of coffee.
What do you like most about your job?
As a project leader I enjoy working on creative solutions with my project teams in order to successfully manage the project and meet customer expectations.
Have you had any particular expectation when you came to Bachem and have these been fulfilled?
For me one of the most important aspects of a desirable job is a good team atmosphere with my colleagues. This expectation has been by far exceeded.
Thank you very much Sandra.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
New Peptide could Improve Treatment for Vision-Threatening Disease-Johns Hopkins Medicine
Endogenous Peptide Lowers Cholesterol-Ludwig-Maximilians-Universität München
TSRI Scientists Find Brain Hormone that Triggers Fat Burning-The Scripps Research Institute
Molecule Shows Ability to Thwart Pathogens’ Genetic Resistance to Antibiotic-Science Daily
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
D. Martin-Sanchez, O. Ruiz-Andres, J. Poveda, S. Carrasco, P. Cannata-Ortiz, M. D. Sanchez-Nino, M. Ruiz Ortega, J. Egido, A. Linkermann, A. Ortiz, A. B. Sanz
Ferroptosis, but Not Necroptosis, Is Important in Nephrotoxic Folic Acid-Induced AKI
J. Am. Soc. Nephrol. 28 (1), 218-229 (2017)
P. Antonietti, B. Linder, S. Hehlgans, I. C. Mildenberger, M. C. Burger, S. Fulda, J. P. Steinbach, F. Gessler, F. Rodel, M. Mittelbronn, D. Kogel
Interference with the HSF1/HSP70/BAG3 Pathway Primes Glioma Cells to Matrix Detachment and BH3 Mimetic-Induced Apoptosis
Mol. Cancer Ther. 16.(1), 156-168 (2017)
L. Li, G. Qian, Y. Zuo, Y. Yuan, Q. Cheng, T. Guo, J. Liu, C. Liu, L. Zhang, H. Zheng
Ubiquitin-dependent Turnover of Adenosine Deaminase Acting on RNA 1 (ADAR1) Is Required for Efficient Antiviral Activity of Type I Interferon
J. Biol. Chem. 291 (48), 24974-24985 (2016)
X. Wang, Z. He, H. Liu, S. Yousefi, H. U. Simon
Neutrophil Necroptosis Is Triggered by Ligation of Adhesion Molecules following GM-CSF Priming
J. Immunol. 197 (10), 4090-4100 (2016)
J. Sosna, S. Philipp, J. Fuchslocher Chico, C. Saggau, J. Fritsch, A. Foll, J. Plenge, C. Arenz, T. Pinkert, H. Kalthoff, A. Trauzold, I. Schmitz, S. Schutze, D. Adam
Differences and Similarities in TRAIL- and Tumor Necrosis Factor-Mediated Necroptotic Signaling in Cancer Cells
