The demand for oligonucleotide therapeutics is booming
Emerging as a new treatment option in rare and orphan disease areas, oligonucleotide therapeutics have matured into a drug class with a broad indication spectrum. The promise of oligonucleotides is making a difference to an increasing number of patients. If you have the products to give patients hope, we can help you get them to market – quickly.
Let’s build a new future in oligonucleotides
If you’ve developed a useful oligonucleotide, you’ll know just how exciting the possibilities are. But to realize those possibilities, you need expertise in process development, GMP manufacturing and meeting regulations. That’s where we can help.
We bring innovation and decades of experience to every stage of the process, boosting your product’s potential with new engineering solutions for tight process control and high throughput and we can get your product to market!

Perfect your oligonucleotide drug
Working with us, you’ll have an exceptional analytical chemistry team on board. Our team has vast experience in developing and validating test methods for large and structurally complicated molecules. Not to mention their expertise in solid-phase synthesis, protecting group chemistry, purification by chromatography, ultra/diafiltration techniques, precipitation and lyophilization.
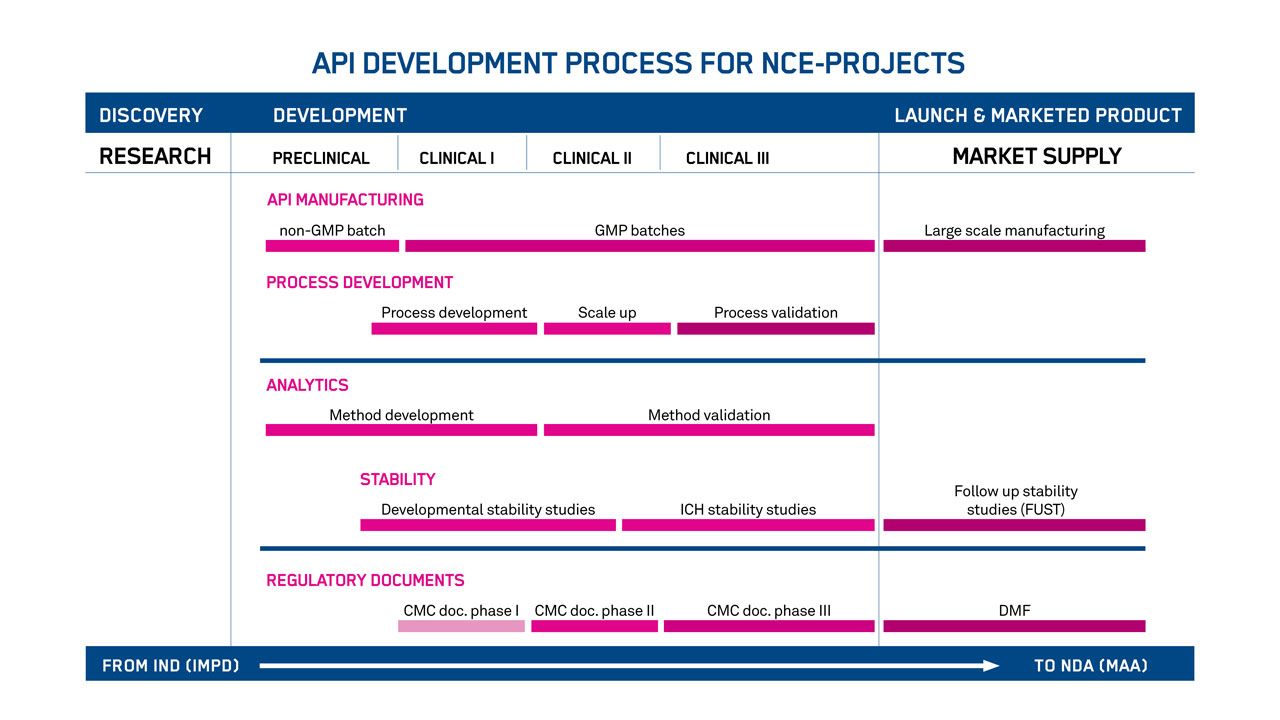
Your products will feel the full benefit of our advanced chemistry, manufacturing, and control (CMC) development services for oligonucleotide-based APIs. It starts with the establishment of scalable manufacturing processes. And it ends with IND and NDA applications supported by our in-house regulatory affairs specialists.
Avoid the risks associated with clinical and commercial milestones. We’ll create a straightforward plan for getting your oligonucleotides to market.
Get some more details on our oligonucleotide services
Why work with us?
- Capacity – Rapid implementation of manufacturing capacities for oligo-based APIs
- Excellence – We offer the highest quality products and services in the industry, with broad knowledge and expertise in peptide development and production.
- Internationally approved – Our facilities are regularly inspected by the FDA, Swissmedic and other prominent regulatory authorities – and we’re fully GMP compliant
- Expertise – Our people are highly skilled and passionate about emerging technologies, but also pride themselves on being reliable and committed to customers.
- Sustainability – We’re convinced that ethical behavior and integrity are essential for long-term business success, so we’re focused on improving our social, economic and environmental sustainability.
The future of oligonucleotide manufacturing
What our customers are saying
Oligonucleotide news

Back to stirred vessels and on to metric tons of oligonucleotides

The Latest Trends in Peptides and Oligonucleotides

How can pharma and biotech benefit from strategic alliances?
Next Oligo events coming up
RNA Leaders Europe Congress 2024
RNA Leaders Europe Congress 2024
Bachem participates at the RNA Leaders Europe Congress 2024, March 13-14 in Basel
Peptide Based Therapeutics Summit
Peptide Based Therapeutics Summit
Bachem is set to attend the Peptide Based Therapeutics Summit, April 23-25, showcasing the forefront of peptide drug conjugates, bicyclic and macro-cyclic peptides, and peptide-based therapeutic innovation. The Summit is quickly establishing itself as a pivotal conferen...
CPhI North America 2024
CPhI North America 2024
Meet Bachem at the CPhI North America at Philadelphia in May 7-9. The event will take place at Pennsylvania Convention Centre.
Oligonucleotide knowledge
Our Knowledge Center has a wide variety of tools and materials for oligonucleotide chemistry.
Oligo calculator
Click here >>
Brochures
Click here >>
White papers
Click here >>
Oligo FAQ
Click here >>
Further topics of interest
Quality &
Regulatory
Click here >>
GMP
Production
Click here >>
About
Bachem
Click here >>
Contact us




