Peptide-based peptide cancer therapeutics
This white paper discusses the potential use of peptides as anticancer drugs highlighting current scenario and future prospects. Some peptides are also used as diagnostic tools for cancer detection. G-protein-coupled receptors are most important targets in drug development. Many of them are overexpressed in tumor cells. Amongst them, the GnRH receptor is the target of a considerable number of GnRH agonists and antagonists used in cancer management. GnRH (gonadotropin-releasing hormone) or LHRH (luteinizing hormone-releasing hormone) is a decapeptide produced in in the hypothalamus and released in a pulsatile fashion into the pituitary portal circulation. Prolonged non-pulsatile administration of LHRH leads to down-regulation of LH and FSH secretion, followed by a suppression of gonadal steroid synthesis.
For this reason, longer-acting GnRH agonists as well as antagonists are used for the treatment of hormone-dependent breast and prostate cancers. Most neuroendocrine tumors show a marked overexpression of somatostatin receptors, especially of sst2, which instigated the development of somatostatin agonists as octreotide. These compounds also play an important role in diagnosis. Bombesin/gastrin-releasing peptide receptors can be overexpressed in malignant cells. Antagonists of these peptides inhibit tumor growth. Active immunization by peptide vaccines is another promising strategy to fight cancer.
Introduction
Cancer is characterized by uncontrolled division of cells and the ability of these cells to invade other tissues leading to the formation of tumor mass, to vascularization and, finally, to metastasis (spread of cancer to other parts of the body). Though angiogenesis (growth of new blood vessels from existing vessels) is a normal and vital process during growth and development, it is also a fundamental step in the transition of tumors from a dormant state to a malignant one. So, angiogenesis inhibitors have been used to suppress tumor cell growth.
Chemotherapy is one of the classical approaches to treat cancer, a cytotoxic agent is delivered to the cancer cells. The main problem with conventional chemotherapy is its inability to administer the correct amount of drug directly to cancer cells without affecting normal cells. Drug resistance, altered biodistribution, biotransformation and premature clearance are also common problems. Targeted chemotherapy and drug delivery techniques are emerging as a powerful method to circumvent such problems.
Modification at position 6 with a d-amino acid yields potent long-acting LHRH agonists.
The “biologics” approach to cancer therapy includes application of proteins, monoclonal antibodies and peptides. Monoclonal antibodies (mAb) and large protein ligands have two major limitations compared to peptides: poor delivery to tumors due to their large size and a dose-limiting toxicity in liver and bone marrow due to nonspecific uptake into the reticuloendothelial system.
The use of such macromolecules has therefore been restricted to vascular targets present on the luminal side of the tumor vessel endothelium and to hematological malignancies. Peptides possess many advantages such as small size, ease of synthesis and modification, they are biocompatible and can penetrate tumor tissue.
Their proteolytic degradation can be conveniently prevented by chemical modifications such as incorporation of D-amino acids or cyclization. Properties of bicyclic peptides are even better and comparable to those of antibody drugs. The peptide drugs currently available on the market can be classified as analogs and antagonists of peptide hormones or tumor targeting agents carrying radionuclides.
LHRH (GnRH) Agonists and Antagonists
The first example for the introduction of peptide drugs into cancer therapy is the use of LHRH (luteinizing hormone-releasing hormone) analogs. Schally et al. developed the first GnRH agonists which later were applied in the treatment of prostate and breast cancer. Since then, peptides such as buserelin, leuprolide, goserelin, histrelin, and triptorelin have been developed and approved in cancer therapy. Depot formulations of these peptides allow for a more efficacious and convenient treatment of patients with prostate cancer.
Administration of these peptides effects a down-regulation of GnRH receptors in the pituitary, leading to an inhibition of follicle-stimulating hormone (FSH) and LH release, and a concomitant decrease in testosterone production. The introduction of LHRH antagonists as cetrorelix resulted in therapeutic improvement over agonists as they cause an immediate and dose-related inhibition of LH and FSH by competitive blockade of the LHRH receptors. To date, many potent GnRH antagonists are available for therapeutic use in patients suffering from prostate cancer. A list of such agonists and antagonists available in the market can be found in Table 1.
| PEPTIDE | SEQUENCE | INDICATION |
|---|---|---|
GnRH | ||
| GONADORELIN | Pyr-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH? | None in cancer therapy |
GnRH Agonists | ||
| BUSERELIN | Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-Arg-Pro-NHEt | Prostate cancer |
| GOSERELIN | Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-Arg-Pro-Azagly-NH? | Prostate and breast cancer |
| HISTRELIN | Pyr-His-Trp-Ser-Tyr-D-His(Bzl)-Leu-Arg-Pro-NHEt | Prostate and breast cancer |
| LEUPROLIDE | Pyr-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt | Prostate and breast cancer |
| TRIPTORELIN | Pyr-His-Trp-Ser-Tyr-D-Trp-Leu-Arg-Pro-Gly-NH? | Prostate and breast cancer |
GnRH Antagonists | ||
| ABARELIX | Ac-D-2-Nal-D-4-Cpa-D-3-Pal-Ser-N-Me-Tyr-D-Asn-Leu-Lys(isopropyl)-Pro-D-Ala-NH? | Prostate cancer |
| CETRORELIX | Ac-D-2-Nal-4-chloro-D-Phe-?-(3-pyridyl)-D-Ala-Ser-Tyr-D-Cit-Leu-Arg-Pro-D-Ala-NH? | Prostate and breast cancer |
| DEGARELX | Ac-D-2-Nal-D-4-Cpa-D-3-Pal-Ser-4-amino-Phe(L-4,5-dihydroorotyl)-4-ureido-D-Phe-Leu-Lys(isopropyl)-Pro-D-Ala-NH? | Prostate cancer |
| OZARELIX | Ac-D-2-Nal-D-4-Cpa-D-3-Pal-Ser-N-Me-Tyr-D-Hci-Nle-Arg-Pro-D-Ala-NH? | Prostate cancer |
| TEVERELIX | Ac-D-2-Nal-D-4-Cpa-D-3-Pal-Ser-Tyr-D-Hci-Leu-Lys(isopropyl)-Pro-D-Ala-NH? | Prostate cancer |
Table1. LHRH agonists and new generation antagonists available in the market.
Somatostatin Analogs in Cancer Therapy
Apart from the use of peptidic LHRH agonists and antagonists for treating cancer, somatostatin analogs are the only approved cancer therapeutic peptides in the market. Potent agonists of somatostatin (SRIF) including octreotide (sandostatin) have been developed for the treatment of acromegaly, gigantism and thyrotropinoma associated with carcinoid syndrome, and diarrhea in patients with vasoactive intestinal peptidesecreting tumors (VIPomas). Lanreotide, another long-acting analog of somatostatin, is used in the management of acromegaly and symptoms caused by neuroendocrine tumors.
Most neuroendocrine tumors (NETs) feature a strong overexpression of somatostatin receptors, mainly of subtype 2 (sst2). Currently, five somatostatin receptor subtypes (sst) are known (sst1-5). The density of these receptors on tumor tissue is vastly higher than on healthy tissue. Therefore, sst are attractive targets for delivery of radionuclides employing appropriately modified somatostatin analogs. Introduced in the late 1980s by Sandoz, [111In-DTPA]-octreotide (pentetreotide, Octreoscan®), rapidly became the gold standard for diagnosis of sst-positive NETs. Numerous peptide-based tumor-imaging agents targeting sst have been developed over the past decades.
Octreoscan® and NeoTect® (technetium-99m-labeled depreotide, cyclo(MePhe-Tyr-D-Trp-Lys-Val-Hcy(CH2CO- β-Dap-Lys-Cys-Lys-NH2)) are the only radiopeptide tracers on the market approved by the FDA. An octreotide scan or octreoscan is a scintigraphic method used to find carcinoids and other types of tumors and to localize sarcoidosis. DTPA-Octreotide, after radiolabeling with indium-111, is injected into a vein and travels through the bloodstream. The radioactive octreotide attaches to tumor cells that have receptors for somatostatin.
The five known somatostatin receptors are attractive targets for tumor diagnosis and therapy
Cyclic chelators as DOTA bind (radio)nuclides as 68Ga, 90Y, or 177Lu more tightly, so (Tyr3)-DOTAoctreotide (DOTATOC, edotreotide) can be used in diagnosis and therapy of NETs. This also holds true for the C-terminal acid, DOTA-octreotate (DOTATATE). Somatostatin agonists vary in receptor selectivity: Lanreotide shows high affinity for sst2 and somewhat less to sst5. Pasireotide, another SRIF agonist, binds less selectively and thus mimics the naturalligand more closely.
Peptide Vaccines
Active immunization seems to be the most promising strategy to treat cancer though many approaches based on the employment of immune cells or immune molecules have been followed. This method of treating cancerous cells relies on vaccines consisting of peptides derived from the amino acid sequence of candidate tumor-associated or specific antigens. Tumor cells express antigens known as tumor-associated antigens (TAAs) that can be recognized by the T-cells of the host’s immune system. A considerable number of TAAs could be identified and characterized.
TAAs can be injected into cancer patients in an attempt to induce a systemic immune response that may result in the destruction of the cancer cells. Any protein/peptide produced in a tumor cell that has mal structure due to mutation can act as a tumor antigen. Such abnormal proteins are produced due to mutations in the corresponding gene. Hence, clinical studies have been initiated to assess the therapeutic potential of active immunization or vaccination with TAA peptides in patients with metastatic cancer.
So far, only a limited number of TAA peptides, mostly those recognized by CD8 (+) T-cells in melanoma patients, have been clinically tested. Several melanoma TAAs have been identified and are being evaluated as peptide-based cancer vaccines in clinical trials around the world. Recent advances in the field of molecular biology have enabled the rapid identification of dozens of candidate TAAs for several important human cancers.
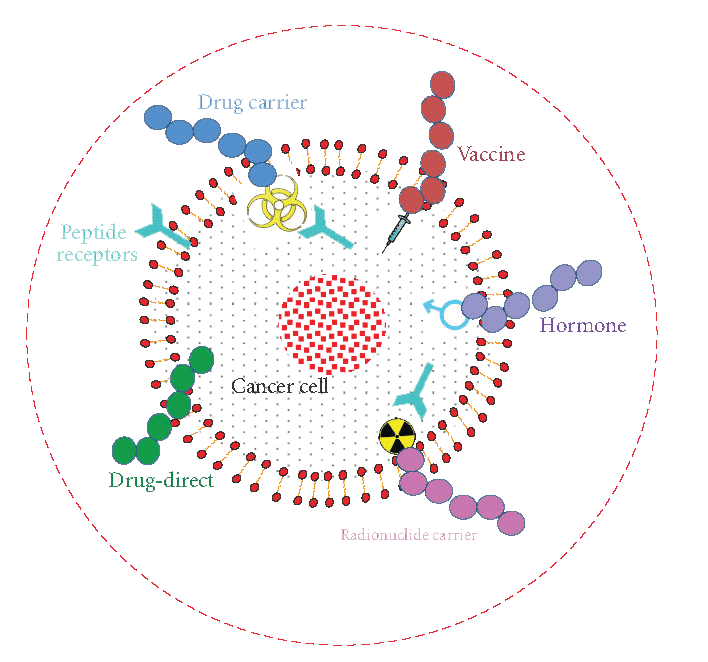
Figure1. Different treatment options of cancer using peptides. Peptides can be used as: anti-cancer drug, cytotoxic drug carrier, vaccine, hormone, radionuclide carrier and drug target (cancer drugs can be targeted towards tumor associated peptides or peptide receptors. (J. Thundimadathil, J. Amino Acids 2012, 13 (2012))
Current Status and Future of Peptide based anti-cancer agents
The application of peptides as direct therapeutic agents, in targeted drug delivery and as diagnostic tools in cancer biology is growing. Among many improvements in targeted and controlled delivery of therapeutics, specifically binding peptides have emerged as the most valuable non-immunogenic approach to target cancer cells. Various cancer treatment options using peptides are summarized in Figure 1.
The RGD peptide iRGD (CRGDKGPDC) is able to specifically recognize and penetrate cancerous tumors but not normal tissues. The development of similar peptides with extraordinary tumor-penetrating properties will definitely make substantial improvements in cancer treatment in future. Chlorotoxin (Bachem product 4044876, a 36 amino acid peptide isolated from scorpion venom) has a higher affinity for glioma cells than for non-neoplastic and normal brain cells.
This preferential binding has allowed the development of new methods for the treatment and diagnosis of brain cancer. Antiangiogenesis as a therapeutic approach led to renewed interest in cilengitide. This integrin inhibitor, a cyclic RGD peptide, is being evaluated as non-small-cell lung cancer therapeutic in clinical trials. Bombesin/gastrin releasing peptide (BN/ GRP) peptides were shown to bind selectively to the G-protein–coupled receptors on the cell surface, stimulating the growth of various malignancies in murine and human cancer models.
Thus, it has been proposed that the secretion of BN/GRP by neuroendocrine cells might be responsible for the development and progression of prostate cancer to androgen independence. GRP is widely distributed in lung and gastrointestinal tracts. It is produced in small cell lung cancer (SCLC), breast, prostatic, and pancreatic cancer, and functions as a growth factor. The involvement of bombesin- like peptides in the pathogenesis of a wide range of human tumors, their function as autocrine/paracrine tumoral growth factors, and the high incidence of BN/GRP receptors in various human cancers prompted the design and synthesis of BN/ GRP receptor (GRPR) antagonists such as RC-3095, RC-3940-II, and RC-3950.
Peptides will make a huge impact in the area of cancer diagnosis and therapy in the near future.
Currently, many researchers are focusing on the development of GHRH (growth hormone releasing hormone – a hypothalamic polypeptide) antagonists as potential anti-cancer therapeutics since GHRH is produced by various human tumors, including prostate cancer, and seems to exert an autocrine/paracrine stimulatory effect on them.
Another promising approach for the therapy of prostate cancer consists of the use of cytotoxic analogues of GnRH, bombesin, and somatostatin, which can be targeted to receptors for these peptides in prostate cancers and their metastases. For example, a potential drug candidate, AEZS- 108 consists of a peptide LHRH, coupled to the chemotherapeutic agent doxorubicin to directly target cells that express GnRH receptors, specifically, prostate cancer cells.
There is a tremendous effort to discover angiogenesis inhibitors, based on polypeptidesas the safest and least toxic therapy for diseases associated with abnormal angiogenesis. A number of ongoing clinical trials in this area focus on peptides derived from: extracellular matrix proteins, growth factors and growth factor receptors, coagulation cascade proteins, chemokines, Type I Thrombospondin domain containing proteins and serpins.
Subscribe to our newsletter
"*" indicates required fields
References
L.J. Hofland et al.
Somatostatin analogs: clinical application in relation to human somatostatin receptor subtypes.
Biochem. Pharmacol. 50, 287-297 (1995)
J.C. Reubi et al.
Somatostatin receptors and their subtypes in human tumors and in peritumoral vessels.
Metabolism 45, 39-41 (1996)
A.V. Schally
Luteinizing hormone-releasing hormone analogs: their impact on the control of tumorigenesis.
Peptides 20, 1247-1262 (1999)
W.A. Breeman et al.
Somatostatin receptor-mediated imaging and therapy: basic science, current knowledge, limitations and future perspectives.
Eur. J. Nucl. Med. 28, 1421-1429 (2001)
S.V. Le et al.
PAC1 and PACAP expression, signaling, and effect on the growth of HCT8, human colonic tumor cells.
Regul. Pept. 109, 115-125 (2002)
C. Borghouts et al.
Current strategies for the development of peptide-based anti-cancer therapeutics.
J. Pept. Sci. 11, 713-726 (2005)
L.K. Kvols and E.A. Woltering
Role of somatostatin analogs in the clinical management of non-neuroendocrine solid tumors.
Anticancer Drugs 17, 601-608 (2006)
R.T. Dorsam and J.S. Gutkind
G-protein-coupled receptors and cancer.
Nat. Rev. Cancer 7, 79-94 (2007)
S. Fister et al.
Gonadotropin-releasing hormone type II antagonists induce apoptotic cell death in human endometrial and ovarian cancer cells in vitro and in vivo.
Cancer Res. 67, 1750-1756 (2007)
L. Vujanovic and L.H. Butterfield
Melanoma cancer vaccines and anti-tumor T cell responses.
J. Cell. Biochem. 102, 301-310 (2007)
F.G. Rick et al.
Agonists of luteinizing hormone-releasing hormone in prostate cancer.
Expert Opin. Pharmacother. 14, 2237-2247 (2013)
F. Goel et al.
LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women.
Cochrane Database Syst. Rev. CD004562 (2009)
A. Hackshaw
Luteinizing hormone-releasing hormone (LHRH) agonists in the treatment of breast cancer.
Expert Opin. Pharmacother. 10, 2633-2639 (2009)
F. Klug et al.
Characterization of MHC ligands for peptide based tumor vaccination.
Curr. Pharm. Des. 15, 3221-3236 (2009)
G. Mezö and M. Manea
Luteinizing hormone-releasing hormone antagonists.
Expert Opin. Ther. Pat. 19, 1771-1785 (2009)
R.M. Myers et al.
Cancer, chemistry, and the cell: molecules that interact with the neurotensin receptors.
ACS Chem. Biol. 4, 503-525 (2009)
E.D. Deeks
Histrelin: in advanced prostate cancer.
Drugs 70, 623-630 (2010)
F. Hohla and A.V. Schally
Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer.
Cell Cycle 9, 1738-1741 (2010)
P.J. Pommerville and J.G. de Boer
GnRH antagonists in the treatment of advanced prostate cancer.
Can. J. Urol. 17, 5063-5070 (2010)
P. Whelan
Triptorelin embonate: a 6-month formulation for prostate cancer.
Expert Opin. Pharmacother. 11, 2929-2932 (2010)
D. Wild et al.
First clinical evidence that imaging with somatostatin receptor antagonists is feasible.
J. Nucl. Med. 52, 1412-1417 (2011)
B.L.R. Kam et al.
Lutetium-labelled peptides for therapy of neuroendocrine tumours.
Eur. J. Nucl. Med. Mol. Imaging 39 Suppl. 1, S103-112 (2012)
K.P. Koopmans and A.W. Glaudemans
Rationale for the use of radiolabelled peptides in diagnosis and therapy.
Eur. J. Nucl. Med. Mol. Imaging 39 Suppl 1, S4-10 (2012)
P. Limonta et al.
GnRH receptors in cancer: from cell biology to novel targeted therapeutic strategies.
Endocr. Rev. 33, 784-811 (2012)
P.W. Moody et al.
Pituitary adenylate cyclase-activating polypeptide causes tyrosine phosphorylation of the epidermal growth factor receptor in lung cancer cells.
J. Pharmacol. Exp. Ther. 341, 873-881 (2012)
P.K. Nanda et al.
Bombesin analogues for gastrinreleasing peptide receptor imaging.
Nucl. Med. Biol. 39, 461-471 (2012)
J. Thundimadathil
Cancer treatment using peptides: Current therapies and future prospects.
J. Amino Acids 2012, 13 (2012)
F. Barbieri et al.
Peptide receptor targeting in cancer: the somatostatin paradigm.
Int. J. Pept. 2013, 926295 (2013)
R.A. Feelders et al.
Pasireotide, a multi-somatostatin receptor ligand with potential efficacy for treatment of pituitary and neuroendocrine tumors.
Drugs Today (Barc.) 49, 89-103 (2013)
E. Harford-Wright et al.
The potential for substance P antagonists as anti-cancer agents in brain tumours.
Recent Pat. CNS Drug. Discov. 8, 13- 23 (2013)
O. Keskin and S. Yalcin
A review of the use of somatostatin analogs in oncology.
Onco Targets Ther. 6, 471-483 (2013)
F.G. Rick et al.
Agonists of luteinizing hormone-releasing hormone in prostate cancer.
Expert Opin. Pharmacother. 14, 2237-2247 (2013)
N.D. Shore et al.
New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists.
Prostate Cancer Prostatic. Dis. 16, 7-15 (2013)
U.W. Tunn et al.
Six-month leuprorelin acetate depot formulations in advanced prostate cancer: a clinical evaluation.
Clin. Interv. Aging 8, 457-464 (2013)
R. Varshney et al.
(68)Ga-labeled bombesin analogs for receptor-mediated imaging.
Recent Results Cancer Res. 194, 221- 256 (2013)
Z. Yu et al.
An update of radiolabeled bombesin analogs for gastrin-releasing peptide receptor targeting.
Curr. Pharm. Des. 19, 3329-3341 (2013)
O. Abdel-Rahman et al.
Somatostatin receptor expression in hepatocellular carcinoma: prognostic and therapeutic considerations
Endocr. Relat. Cancer 21, R485-493 (2014)
A. Accardo et al.
Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs.
Int. J. Nanomedicine 9, 1537-1557 (2014)
V. Ambrosini et al.
The use of gallium-68 labeled somatostatin receptors in PET/CT imaging.
PET Clin. 9, 323-329 (2014)
R. Baldelli et al.
Somatostatin analogs therapy in gastroenteropancreatic neuroendocrine tumors: current aspects and new perspectives.
Front. Endocrinol. (Lausanne) 5, 7 (2014)
N.J. Carter and S.J. Keam
Degarelix: a review of its use in patients with prostate cancer.
Drugs 74, 699-712 (2014)
K. Mander et al.
Advancing drug therapy for braintumours: a current review of the proinflammatory peptide Substance P and its antagonists as anti-cancer agents.
Recent Pat. CNS Drug Discov. 9, 110-121 (2014)
C. Morgat et al.
Targeting neuropeptide receptors for cancer imaging and therapy: perspectives with bombesin, neurotensin, and neuropeptide-Y receptors.
J. Nucl. Med. 55, 1650-1657 (2014)
J.W. Moul
Utility of LHRH antagonists for advanced prostate cancer.
Can. J. Urol. 21, 22-27 (2014)
R. Coveñas and M. Muñoz
Cancer progression and substance P.
Histol. Histopathol. 29, 881-890 (2014)
M. Muñoz and R. Coveñas
Involvement of substance P and the NK-1 receptor in pancreatic cancer.
World J. Gastroenterol. 20, 2321-2334 (2014)
B. Tang et al.
Vasoactive intestinal peptide receptor-based imaging and treatment of tumors (Review).
Int. J. Oncol. 44, 1023-1031 (2014)
Z. Blumenfeld and A. Evron
Preserving fertility when choosing chemotherapy regimens – the role of gonadotropin-releasing hormone agonists.
Expert Opin. Pharmacother. 16, 1009-1020 (2015)
L. Dardevet et al.
Chlorotoxin: a helpful natural scorpion peptide to diagnose glioma and fight tumor invasion.
Toxins (Basel) 7, 1079-1101 (2015)
M. Muñoz and R. Coveñas
Targeting NK-1 Receptors to Prevent and Treat Pancreatic Cancer: a New Therapeutic Approach.
Cancers (Basel) 7, 1215-1232 (2015)
Y. Nishimura et al.
Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses.
Cancer Sci. 106, 505-511 (2015)
J. Yang et al.
Composite peptide-based vaccines for cancer immunotherapy (Review).
Int. J. Mol. Med. 35, 17-23 (2015)
