MEET US AT THE 4TH CHEMICAL LIGATION MEETING

The 4th edition of the symposium entitled «Challenges and prospects in chemoselective ligations: from protein synthesis to site-specific conjugation» will be held in Orléans (France), from January 27 – 29, 2020. This three-day international conference organized under the auspices of the French Peptide and Protein Group (GFPP) will cover the different aspects of chemical ligation in a broad sense, including bioorthogonal chemistry, bioconjugation, late-stage peptide modification and protein chemical synthesis, from methodology development to biological applications.
Bachem supports its customers in the pursuit of groundbreaking discoveries that further scientific advances, particularly in the field of medicine. With a record of accomplishment in custom synthesis projects, the high quality of our peptides for research and development projects and our capacity to upscale the production of simple and modified peptides, we are the Pioneering Partner for Peptides.
Dr. Stéphanie Henriot, Sales Manager Custom Synthesis at Bachem, is excited to meet with you, learn your needs for peptides and discuss how Bachem can assist to advance your research. We invite you to drop by our booth or to contact us to schedule a meeting in advance.
We look forward to meeting you in Orléans!
FLUORESCENTLY-LABELED PEPTIDES
With the advancement of FRET-based enzyme substrates, the need has arisen for more complex fluorescently labeled peptides. In order to facilitate the design and synthesis of these peptides, a variety of different synthetic techniques have been developed.
There are three main functional groups in a generic peptide chain, which allow the attachment of dye labels and require different precursors for the attachment of the dye. These functional groups are
- Amino groups (N-terminus, Lys)
- Thiol groups (Cys)
- Carboxyl groups (C-Terminus, Asp, Glu)
Attachment to Amino Groups
As in standard SPPS or LPPS, primary amines can react with chromophores and fluorophores bearing a carboxylic acid functionality to form an amide bond. In practice, this is usually accomplished by reacting the free amine group with the succinimidyl ester of the dye molecule. These esters represent an activated form of the carboxylic acid, generated by the reaction with N-hydroxy succinimide (NHS). Common examples of such activated acids are 6-carboxyfluorescein succinimidyl ester (CFSE) and the NHS-esters of rhodamine dyes (see Fig 1).
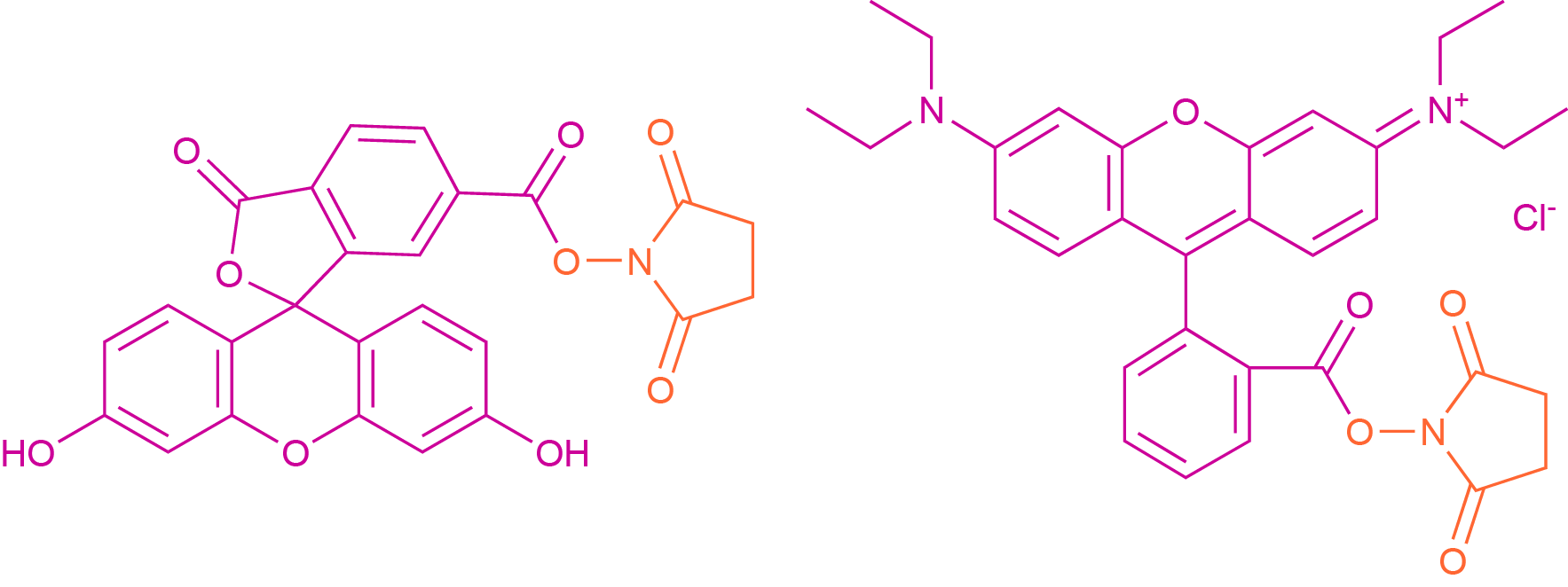
Fig. 1 Reactive Precursors of 6-Carboxyfluorescein (left) and Rhodamine B (right). The dye molecule is colored in magenta, while the leaving group is colored orange.
Apart from its NHS-ester, the fluorescein core can also be attached using its isothiocyanate (FITC) precursor. The isothiocyanate reacts with the nucleophilic primary amine under formation of a thiourea. If FITC is to be used in SPPS, a linker (usually β-Ala or ε-Ahx) has to be inserted between the N-terminus and the dye, since a direct labeling causes the elimination of the N-terminal FITC together with the N-terminal amino acid in an Edman reaction during the final TFA cleavage (see Fig 2).
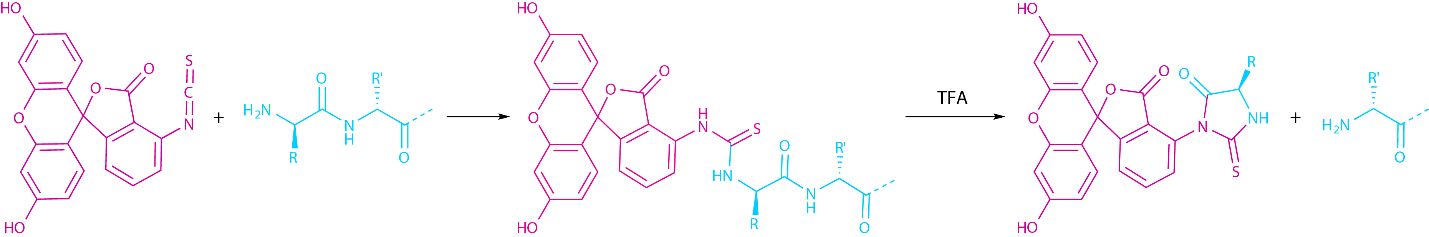
Fig. 2 Edman degradation, which occurs if FITC is attached without a spacer during SPPS.
Some common dye-labels bound to side-chain amino groups are commercially available as building blocks for LPPS and SPPS, such as the fluorophore Abz (Fmoc-Lys(retro-Abz-Boc)-OH) or the common quencher 2,4-dinitrophenol (Dnp) (e.g. Fmoc-Dap(Dnp)-OH or Fmoc-Lys(Dnp)-OH).
Attachment to Thiol Groups
The thiol group of a cysteine residue reacts with maleimides and iodoacetamides with differing selectivity. Whereas iodoacetamides can also bind to methionine or histidine residues, cysteines can be very selectively conjugated to maleimides. Because of this, many commercial dyes are available with a maleimide linker, which reacts in a Michael-type addition reaction with the thiol group (see Fig. 3).
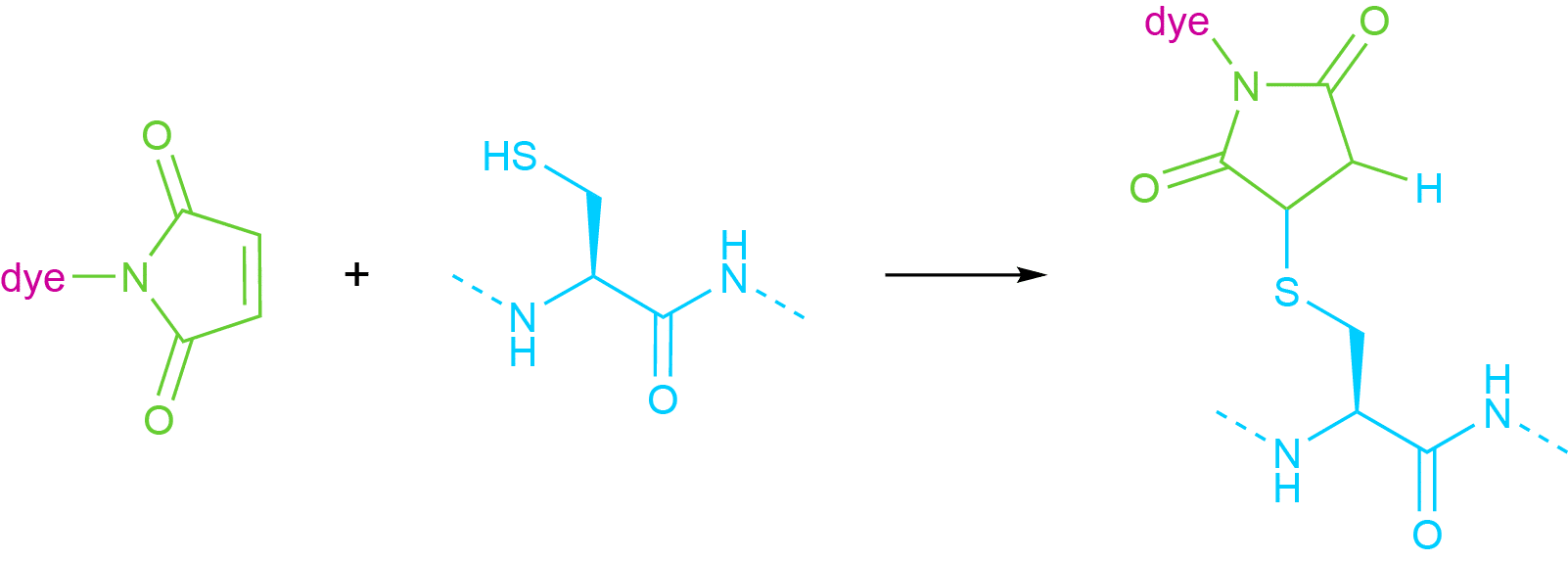
Fig 3. Attachment of a Generic Dye Molecule to a Thiol Group Using a Maleimide Linker.
This reactivity is also exploited in the labeling of larger biomolecules with fluorophores. A rather new development are the so-called FlAsH-probes. These fluorophores contain two arsenic atoms. Each of them binds to two thiol moieties. The dye only turn fluorescent when bound to the tetracysteine motif sequence. The specific recognition sequence allows for very selective labeling (see Fig. 4).
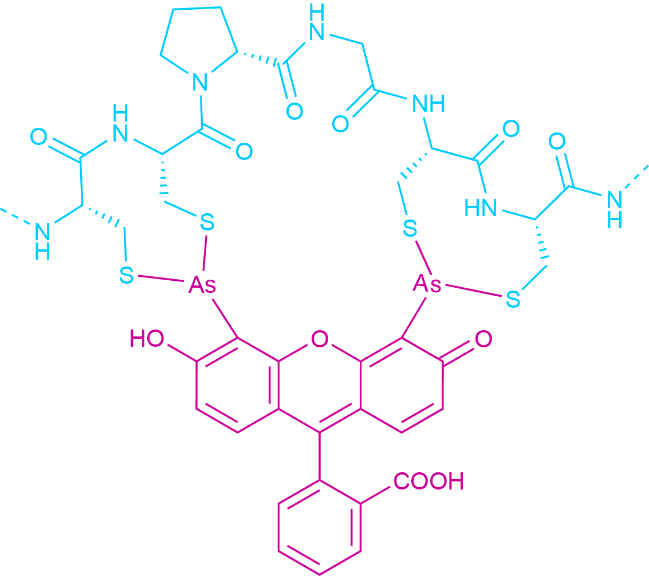
Fig.4 FlAsH Probe Bound to its Minimal Recognition Sequence (CCPGCC).
Attachment to Carboxyl Groups
Coupling to a carboxyl group can be regarded as a reversal of the situation described for the coupling to an amino group. A chromophore or fluorophore bearing a free amine group can be coupled to a sufficiently activated free carboxyl group contained in a peptide chain. However, there are some particularities, which have to be considered. In SPPS, coupling to the C-terminus of a peptide chain can in general not be performed on resin, since the C-terminus is already bound to the solid support. In general, such a coupling is performed in solution phase after cleavage from the resin. The activation of the carboxylic acid is generally not achieved by the formation of an isolatable succinimidyl ester but rather as in SPPS/LPPS by the in situ formation of active esters. A special case are dye labels, which bear a carboxyl and an amino function. Suitably N-protected derivatives of such compounds can be coupled to a resin and the peptide chain can be synthesized on this resin via classical SPPS (see Fig.5).
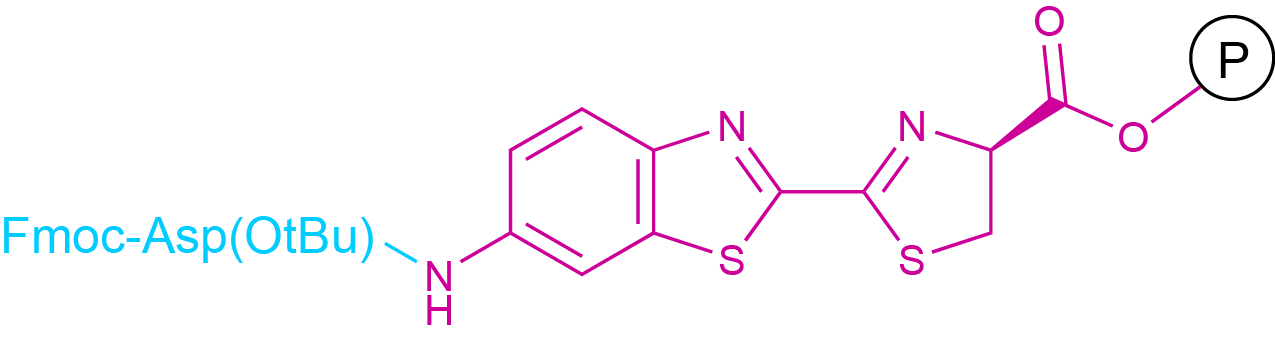
Fig 5. An Example for Polymer-bound D-Aminoluciferin, usable for Fmoc-SPPS.
Unspecific Labeling
The aforementioned attachments can only be done selectively if only one attachment point is available. For instance, the N-terminus of unprotected peptides can be easily labeled with a fluorophore, if the sequence does not contain lysine or other amino acids bearing side-chain amino groups. If this is not the case, either selective protection/deprotection or unspecific labeling can be employed.
Unspecific labeling means that the reaction site at which the dye label is attached to is not clearly determined and the product is a mixture of all possible reaction products. This approach is especially attractive if the unprotected peptide chain is already available, e.g. as a commercially available product. However, it is unsuitable for the synthesis of FRET-substrates, since the position within the peptide chain of acceptor and donor is very important for an effective FRET-contact, as stated before.
References
Monograph «FRET Substrates»
M.Jullian, A.Hernandez, A.Maurras, K.Puget. M.Amblard, J.Martinez, G.Subra, N-Terminus FITC labeling of peptides on solid support: the truth behind the spacer. Tetrahedron Letters 2009, 50, 260-263.
N.Jing, L.Hongyan, S.Hongzhe, Recognition of proteins by metal chelation-based fluorescent probes in cells, Frontiers in Chemistry 2019, 7, 560.
A.K.Kovács, P.Hegyes, G.J.Szebeni, L.I.Nagy, L.G.Puskás, G.K.Tóth, Synthesis of N-peptide-6-amino-D-luciferin conjugates, Frontiers in Chemistry 2018, 6, 120.
K.Renault, J.W. Fredy, P.-Y.Renard, C.Sabot, Covalent modification of biomolecules through maleimide-based labeling strategies, Bioconjugate Chemistry 2018, 29, 2497-2513.
H.Sahoo, Fluorescent labeling techniques in biomolecules: A flashback, RSC advances 2012, 2, 7017-7029.
FLUORESCENT PEPTIDES IN CLINICAL DEVELOPMENT
Fluorescently labeled peptides have the potential to make a positive impact on image-guided surgery and disease diagnosis. A technique of increasing interest involves the use of fluorescent peptide probes that bind or become modified by a molecular target overexpressed at the disease site. In the area of oncology, several fluorescently labeled peptides are already in the clinical stages of development as shown in Table 1.
| Product Name | Companies Involved | Highest Phase | Condition/Application |
|---|---|---|---|
| AVB620 | Avelas Biosciences Inc. | III | Breast Cancer |
| BLZ-100 | Blaze Bioscience Inc. | III | Pediatric Central Nervous System Tumor |
| EMI-137 | Edinburgh Molecular Imaging, University Medical Center Groningen | II | Barrett Esophagus, Esophageal Cancer, Dysplasia in Barrett Esophagus, Papillary Thyroid Cancer, Lymph Node Metastases, Colon Cancer |
| ABY-029 | Dartmouth-Hitchcock Medical Center, Affibody AB, LI-COR Biosciences, University of Alabama at Birmingham (UAB) Vector Production Facility | I | Glioma, Head and Neck Cancer, Sarcoma |
| KCC Peptide | University of Michigan, Olympus Corporation | I | Colon Polyps, Colorectal Cancer, Inflammatory Bowel Disease |
| KSP-910638G Heptapeptide | University of Michigan, Edinburgh Molecular Imaging Ltd | I | Barrett Esophagus |
| LS301 | Washington University School of Medicine | I | Breast Cancer, Pancreatic Cancer, Liver Cancer, Gastric Cancer, Gastrointestinal Stromal Cancer, Metastatic Cancer |
| QRH-882260 Heptapeptide | University of Michigan | I | Colon Cancer Prevention, Barrett Esophagus, Cholangiocarcinoma |
Fluorescently labeled peptides have the potential to make a positive impact on image-guided surgery and disease diagnosis. A technique of increasing interest involves the use of fluorescent peptide probes that bind or become modified by a molecular target overexpressed at the disease site. In the area of oncology, several fluorescently labeled peptides are already in the clinical stages of development as shown in Table 1.
Phase III Candidates
Avelas Biosciences is developing AVB620 as a real-time surgical diagnostic agent for breast cancer. AVB620 is a fluorescent protease-activated peptide that detects, marks, and diagnoses cancer. The product utilizes cell-penetrating peptides to deliver a fluorescent marker specifically to cancer cells. AVB620 is administered by intravenous injection before surgery and imaging is performed with a fluorescence-imaging camera to identify critical cancer margins and stage lymph nodes (1). In 2018, Avelas presented initial results from the first part of a Phase II clinical trial of AVB620 that demonstrated the potential of using AVB620 during surgery to identify malignant tissue and lymph nodes (2).
BLZ-100 (tozuleristide) is being developed by Blaze Bioscience for use as an imaging agent during cancer surgery for a range of cancer types including brain, breast, prostate, lung, colorectal, skin cancer, and sarcomas. BLZ-100 is used to “paint” tumors in order to guide surgeons during cancer removal. The tumor paint consists of chlorotoxin, a tumor penetrating peptide, conjugated to indocyanine green dye. In April 2016, Blaze completed a Phase I study of BLZ-100 in adult subjects with glioma undergoing surgery (3). BLZ-100 is currently being evaluated in a Phase II/III clinical study in pediatric central nervous system tumors (4).
Phase II Candidate
Edinburgh Molecular Imaging has developed a fluorescent optical imaging agent, EMI-137, that specifically targets the biomarker c-Met. The tracer is a small peptide labeled with a fluorophore. In 2017, Edinburgh Molecular Imaging announced that it initiated a Phase IIb clinical trial of EMI-137 in patients with suspected colorectal cancer (5).
Phase I Candidates
Dartmouth is collaborating with Affibody AB, LI-COR Biosciences, and the University of Alabama at Birmingham (UAB) Vector Production Facility to develop ABY-029, a fluorescence imaging and contrast agent for the detection of tumor cells during surgery. ABY-029 binds to cancer cell receptors and highlights tumors (6). The product is an IRDye® 800CW Maleimide labeled Affibody peptide. Dartmouth currently has three clinical trials of ABY-029 waiting for approval from the U.S. Food and Drug Administration of eIND amendments (7) (8) (9).
KCC Peptide contains seven amino acids and is attached to FITC (Fluorescein isothiocyanate). Researchers at the University of Michigan are developing KCC Peptide for use during colonoscopy procedures. The team’s technique involves spraying the fluorescent peptide onto the colon during colonoscopy so that the peptide will illuminate any abnormal or pre-cancerous areas when a special light is used in the scope. In 2016, the University of Michigan completed a Phase Ib study with KCC Peptide to determine if the peptide glows sufficiently (10).
The University of Michigan is also developing KSP-910638G, a fluorescently labeled peptide that consists of a heptapeptide attached via a five amino acid linker to a near-infrared fluorophore, IRDye® 800CW. This peptide is specific for human epithelial growth factor receptor 2 (HER2) (11). In 2019, the University of Michigan completed a Phase I study of KSP-910638G for detection of Barrett’s neoplasia in the esophagus (12).
LS301 is being developed by Washington University School of Medicine for use in fluorescence-guided cancer surgery. LS301 consists of a near-infrared fluorescent dye called cypate and a D-cysteine containing cyclic octapeptide. The peptide component of LS301 targets and binds integrin receptors expressed on tumor cells. A Phase I clinical trial is planned that will use LS301 during partial mastectomy and sentinel lymph node biopsy for breast cancer in order to evaluate LS301 uptake in tumors (13). In addition, a Phase I study is planned to evaluate LS301 uptake in patients undergoing liver, pancreas, or gastric surgery (14).
In addition, the University of Michigan is recruiting participants for a Phase I study of QRH-882260 Heptapeptide, a peptide attached via a five amino acid linker to the near-infrared fluorophore Cy5. The study will test the safety and efficacy of administering QRH-882260 Heptapeptide to patients undergoing clinically indicated endoscopic retrograde cholangiopancreatography (ERCP) for the evaluation of biliary disorders (15).
Conclusion
Fluorescently labeled peptides are valuable tools for molecular imaging and over the past decade, more fluorescent peptide probes have been entering clinical trials. To support the development of labeled peptides for research and clinical applications, Bachem provides a one-stop shop for the custom synthesis service of labeled peptides and amino acids. For custom synthesis, please contact our Custom Synthesis team to request a quote for your labeled peptide or amino acid.
References
1) AVB-620, Avelas Biosciences (2017)
2) Avelas Biosciences Presents Phase II, Period 1 Data in Poster Session at San Antonio Breast Cancer Symposium, Avelas Biosciences (2018)
3) Medtrack (2017)
4) Study of Tozuleristide and the Canvas Imaging System in Pediatric Subjects With CNS Tumors Undergoing Surgery, ClinicalTrials.gov (2019)
5) Edinburgh Molecular Imaging Commences Phase II European Cancer Trial, Edinburgh Molecular Imaging (2017)
6) Dartmouth Wins FDA Approval for Aid to Guide Cancer Surgery, Dartmouth News (2017)
7) A Microdose Evaluation Study of ABY-029 in Recurrent Glioma (ABY-029), ClinicalTrials.gov (2019)
8) A Microdose Evaluation Study of ABY-029 in Primary Sarcoma, ClinicalTrials.gov (2019)
9) A Microdose Evaluation Study of ABY-029 in Head and Neck Oncology Surgery, ClinicalTrials.gov (2019)
10) Study of KCC Peptide Application in the Colon, ClinicalTrials.gov (2017)
11) NCI Drug Dictionary, National Cancer Institute (2019)
12) Study of Multiplexed Heptapeptides for Detection of Neoplasia in the Esophagus, ClinicalTrials.gov (2019)
13) Real-time Intraoperative Breast Cancer Visualization for Margin Assessment, ClinicalTrials.gov (2019)
14) LS301 Uptake in Tumors of Patients Undergoing Liver, Pancreas, or Gastric Surgery, ClinicalTrials.gov (2019)
15) Fluorescence QRH-882260 Peptide Imaging in the Bile Duct, ClinicalTrials.gov (2018)
MEET BACHEM: IVANA MITROVIC
What is your official job title at Bachem?
I work on a 50% base as Sales Representative Inside Sales at Bachem.
How long have you been with Bachem? Where did you work before Bachem?
I started working at Bachem in August 2015 as an apprentice. After finishing the apprenticeship, I joined the Inside Sales Team in 2018.
Briefly, what do you do at Bachem?
I am responsible for inquiries, quotation and order processing of our catalog items. Everyone in our team has regional responsibilities. In addition, I take care of the commercial apprentices when they join our Inside Sales Team.
What is your academic background/degrees or training?
I am currently completing the commercial mature. I would like to start studying Business Administration at the FHNW next year in September.
What do you like to do outside of work?
In my free time, I like to spend time with my friends and family and going to the gym
What makes a perfect day for you?
A perfect day is lying at the beach, drinking a cocktail and watching the sunset.
Thank you very much Ivana.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Metallohelices emulate antimicrobial peptides-Chemistry World
Developing new delivery tools for gene editing-Science Daily
Tapping of cell signaling reveals orphan receptors’ connections-Genetic Engineering News
New approach uses light to stabilize proteins for study-Science Daily
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
H.Minami et al.
Emicizumab, the bispecific antibody to factors IX/IXa and X/Xa, potentiates coagulation function in factor XI-deficient plasma in vitro.
Journal of Thrombosis and Haemostasis 17, 126-137 (2019)
M.Prunk et al.
Increased cystatin F levels correlate with decreased cytotoxicity of cytotoxic T cells.
Radiology and Oncology 53, 57-68 (2019)
H.Tang et al.
Insight into subtilisin E-S7 cleavage pattern based on crystal structure and hydrolysates peptide analysis.
Biochemical and Biophysical Research Communications 512, 623-628 (2019)
M.Wartenberg et al.
Imaging of extracellular cathepsin S activity by a selective near infrared fluorescence substrate-based probe.
Biochimie 166, 84-93 (2019)
