ALZHEIMER`S DISEASE – A REVIEW
Alzheimer’s disease (AD) is the most common type of dementia and accounts for an estimated 60–80% of the reported cases. It is estimated that over 35 million people worldwide have dementia, and this figure is expected to triple by 2050 with a trend of an aging global population. Dementia is characterized by a loss or decline in memory and other cognitive abilities. AD is an irreversible, progressive neurodegenerative disorder. A worldwide struggle is under way to find new treatments and therapies to prevent, cure or even slow the progress of Alzheimer’s disease. The presence of senile plaques, neurofibrillary tangles, neutrophil threads, Beta-Amyloid (Aβ) peptide deposits, and selective loss of neurons are some of the distinctive characteristics of the disease.
The most common feature of the disease is amyloid plaque deposits. Initially Aβ starts as a solitary molecule and tends to come together in the form of clusters which are still soluble and are capable of free travel in the brain, and finally clusters form plaques that are hallmark of AD. Studies show that Aβ has strong binding affinity to the receptors on nerve cells triggering an intracellular process that erodes their synapses with other nerve cells. Aβ are chemically “sticky” and gradually build up into plaques. Aβ peptides are proteolytically cleaved from the membrane bound amyloid precursor protein (APP) which are major constituents of these deposits. The peptides result from the APP, which are being cut by certain enzymes to yield Aβ. Aβ molecules can aggregate to form flexible soluble oligomers which may exist in several forms. It is now believed that certain misfolded oligomers act as seeds, can induce other Aβ molecules to also take the misfolded oligomeric form, leading to a chain reaction. The seeds or the resulting amyloid plaques are toxic to nerve cells. Plaques and tangles tend to spread through the cortex in a predictable pattern as AD progresses. The familial AD genetic evidence shows relation between metabolism of Aβ and AD disease. This progressive brain disorder that damages and eventually destroys the brain cells, leading to dementia and decline in other brain functions, eventually leading to the death of brain cells.
Therefore Alzheimer’s is fatal, and unfortunately so far there is no cure. Alzheimer’s brain abnormalities, including but not limited to:
- Plaques (microscopic clumps of a protein fragment called beta-amyloid (Aβ). These sticky brain plaques are known to consist mainly of Aβ and are known to build up in brain many years before the actual Alzheimer’s symptoms start.
- Tangles, twisted microscopic strands of the protein tau. Tangles destroy a vital cell transport system made of proteins.
- Loss of connections among brain cells responsible for memory, learning and communications. These connections, or synapses, transmit information from cell to cell.
- Inflammation, triggered by body’s immune system
- Eventual death of brain cells and severe tissue shrinkage leading to the brain lesions.
Aβ plays crucial role in AD, and its high concentration (Nano to micro molar) in the brain can cause neuronal death. Pica molar concentration of Aβ slow down the memory and learning process. The Aβ peptide levels in the brain are dynamically and directly influenced by synaptic activity. Low amounts of Aβ, could work as antioxidants, due to its ability to capture redox metals, such as Cu, Fe and Zn, and thus, preventing their participation in redox cycling with other ligands. Aβ peptide has neurotrophic properties and it is hence responsible for the growth and survival of neurons, in the modulation of synaptic function and defense against oxidative stress. The physiological concentrations of Aβ favor the learning and memory processes.
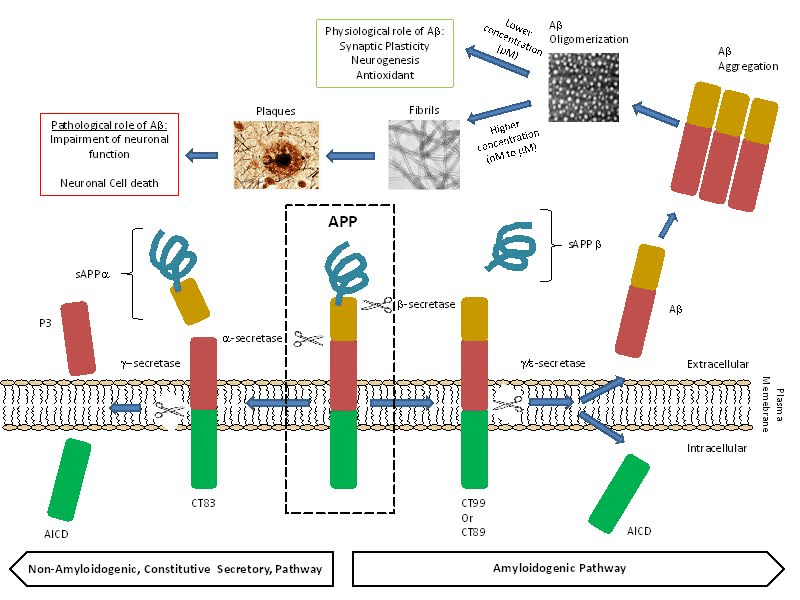
Image courtesy: M. del C. Cárdenas-Aguayo, M. del C. Silva-Lucero, M. Cortes-Ortiz, B. Jiménez-Ramos, L. Gómez-Virgilio, G. Ramírez-Rodríguez, E. Vera- Arroyo, R. Fiorentino-Pérez, U. García, J. Luna-Muñoz and M.A. Meraz-Ríos DOI: 10.5772/57398; click here for details.
Role of Beta Amyloid in the early detection of Alzheimer`s disease
The role of Aβ40 and Aβ42 peptides in the early pathogenesis of the AD has been frequently emphasized in the literature. These two peptides have received tremendous interest in modern AD research. Aβ40 and Aβ42 are major products of the proteolytic cleavage of multi domain integral membrane type-1 protein, APP which play important role in cell adhesion, neuronal mobility and transcriptional regulation. Among the two peptides, Aβ42 is known to be more prone to aggregation than Aβ40, even though they only differ in two (IA) amino acid residues at the C-terminal end. The metabolism of APP involves proteases/secretase processing to yield intra and extra cellular fragments that play important role in synaptic transmission and neuronal plasticity. Amyloidogenic pathway is responsible for beta amyloids peptides through the action of beta and gamma secretases.
Aβ insoluble aggregates in the brain are hallmark of AD, these peptides do exists as soluble aggregates as well. In vitro experiments have obtained fibrillar and different non-fibrillar aggregates. Hexafluoroisopropanol (HFIP) has been used as solvent to dissolve Aβ and other amyloidogenic peptides. HFIP has been used to obtain in vitro a highly ordered fibrillary and variety of non-fibrillar aggregates of beta amyloids. Studies at CSIR/CCMB-India shows the dissolution of Aβ40 and Aβ42 in HFIP and the drying results in highly ordered aggregates. The observed alpha helical confirmation is not stable for prolonged periods. Once the Aβ40, and Aβ42 are incubated in HFIP for longer period of time and eventually dried led to structural transition from alpha helical to beta helical conformation. Then the peptides tend to form short fibrous aggregates that further assemble into highly ordered ring like structures leading to enhancement of thioflavin T fluorescence. The researchers at CSIR-CCMB conclude that dissolution of Aβ40 and Aβ42 or other amyloidogenic fragments in HFIP results formation of structures that are like annular amyloid.
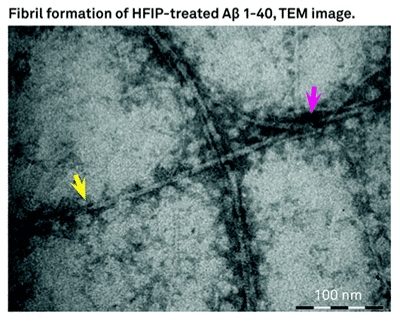
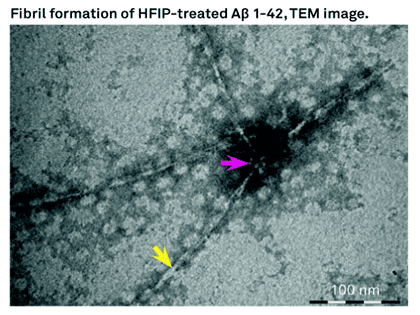
Bachem offers H-7442 and H-7438 which are Beta Amyloid (1-42) and (1-40) peptides treated with HFIP.
Till date it has only been possible to detect AD in late stage when there has already been significant damage to brain. Aβ can be detected in cerebrospinal fluid through a lumbar region puncture and PET (Positron emission tomography) scans. Both being expensive and not easily available everywhere.
Beta amyloid build-up in the eyes of patients in early years has become a biomarker for this disease. The peptide build-up begins 15-20 years before the Alzheimer’s symptoms appear. Studies were conducted and concluded to find significant correlation of the levels of Aβ build-up in eyes and the Aβ build-up in the brain. An abstract prepared by the scientists for AAIC 2014 gives the results for 40 participants out of 200 totals in the study.
References:
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1978067/#R2
http://www.alz.org/braintour/plaques.asp
http://med.stanford.edu/news/all-news/2013/09/scientists-reveal-how-beta-amyloid-may-cause-alzheimers.html
http://www.jbc.org/content/278/13/11612
http://www.ncbi.nlm.nih.gov/pubmed/22252985
