Experience unparalleled quality and reliability with Bachem’s manufacturing facilities in the U.S. and Switzerland. Production is meticulously aligned with the Good Manufacturing Practice (GMP), standards of regulatory agencies such as the Food and Drug Administration (FDA), and the Swissmedic (Switzerland).
Bachem’s production plants are supplied with state-of-the-art equipment for solvent supply, peptide synthesis, purification and isolation of active ingredients and intermediates. And with GMP-qualified equipment and validated cleaning processes, you can rest assured that you get consistent excellence in every batch.
Our recipe for success is a close working relationship with our clients. Through close collaboration, we customise process development packages, meeting ambitious timelines and delivering phase-appropriate, high-quality products with unparalleled efficiency.
Trust Bachem to elevate your production standards and drive success in every aspect of your project.
Elevating Manufacturing Excellence with Bachem
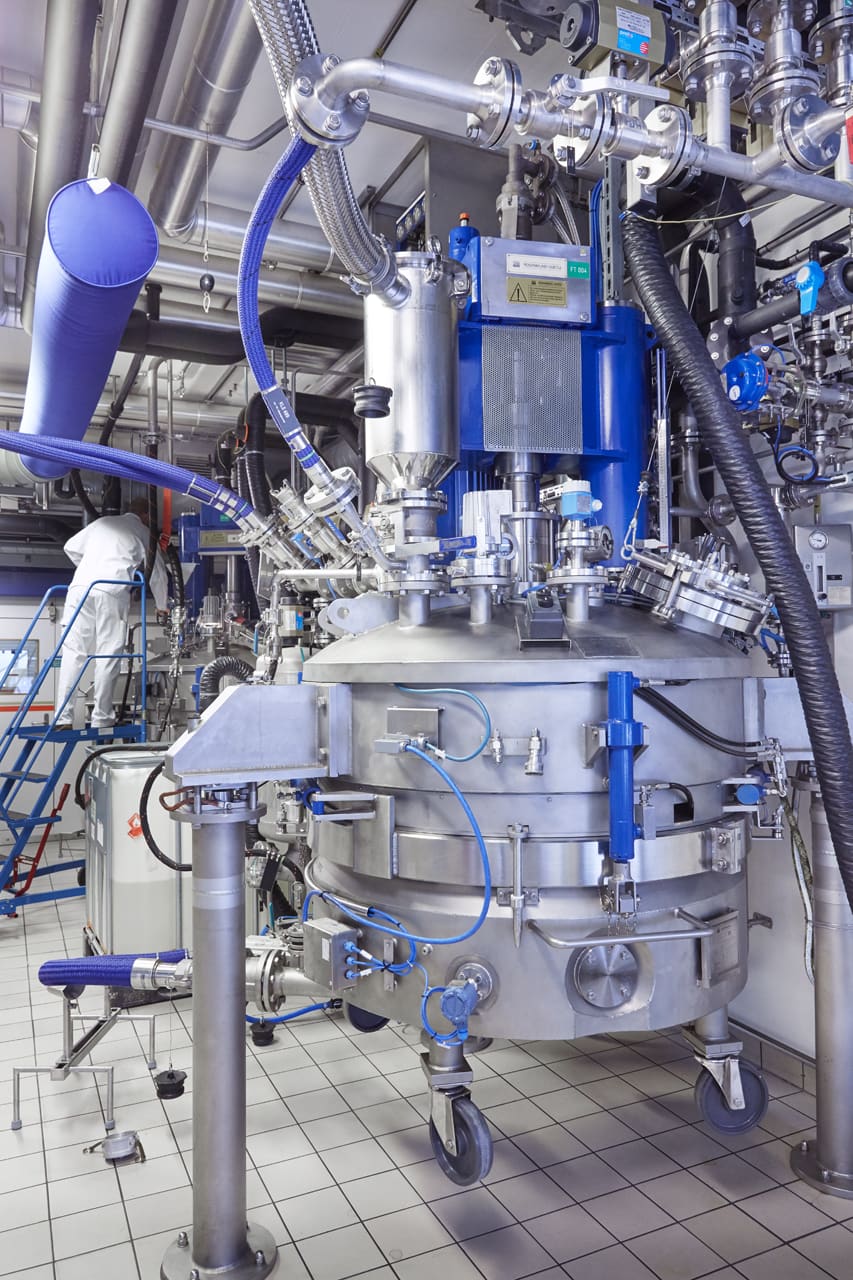
Peptide and oligonucleotide synthesis (“Upstream”)
Our versatile capabilities, expert guidance, and commitment to quality ensure seamless scalability, advanced equipment utilisation, and optimised efficiency throughout the production process. In fact, when you work with Bachem, you benefit from:
- Versatile production capabilities: Including solution-phase and solid-phase synthesis reactors, as well as innovative techniques like Molecular Hiving™.
- Seamless scalability: Effortless scalability with solution-phase reactors up to 8000 litres and solid-phase peptide synthesis (SPPS) reactors up to 1000 litres. Larger options are also in development, with a new facility in Bubendorf offering sizes up to 3000 litres.
- Advanced equipment: Trust in our GMP-qualified equipment, including OligoPilot™ synthesisers and custom-made cleavage and deprotection equipment trains, ensuring precise control and safety in handling hazardous reagents.
- Optimised efficiency and quality: Our advanced automation and data management systems, such as a Manufacturing Execution System (MES) and process data historian, optimise efficiency and guarantee top-notch quality.
- Commitment to quality: Following Quality by Design (QbD) principles, integrating in-process controls (IPC) and process analytical technology (PAT), we consistently deliver high-quality results.
Peptide and oligonucleotide purification and isolation (“Downstream”)
Bachem is dedicated to continually upgrading and modernising its purification equipment to meet the growing demand for bulk peptide and oligonucleotide pharmaceuticals. As well as this, our team will provide you with:
- Advanced purification methods: Utilise a range of advanced purification techniques, including preparative high-performance liquid chromatography (HPLC), ion exchange (IEX), size-exclusion chromatography (SEC), and ultra-filtration (UF/TFF).
- Scalable manufacturing: Our equipment enables highly efficient or continuous manufacturing of ultra-pure products in multi-kg quantities per lot.
- Precise control and scalability: Dynamic axial compression (DAC) stainless steel columns up to 60 cm in diameter ensure optimal separation phase performance.
- Stringent microbiological control: ISO 8 and ISO 7 clean rooms supplied with HEPA-filtered air maintain stringent microbiological control standards.
- Safety measures: Down-flow booths minimise microbial contamination, and highly active pharmaceutical ingredients are handled in safety workbenches or isolators up to OEB level 4.
- Controlled isolation: Carefully controlled isolation processes, including precipitation, crystallisation, and lyophilisation of intermediates and final API, ensure predefined physicochemical properties.
- Multiple lyophilisers for quality assurance: Multiple lyophilisers of various sizes guarantee product integrity and quality across every batch.
Benefit from our small molecule manufacturing
Bachem has capabilities for the production of GMP small molecules at the site in Vionnaz. We can also offer your company:
- Process development
- Chiral synthesis
- Heterocyclic chemistry
- Metal-catalysed reactions
- Hydrogenations
- Oxidations and reductions using various reagents
- Enzymatic reactions
- High-pressure reactions
What is GMP?
GMP, or Good Manufacturing Practices, are essential standards mandated by regulatory agencies such as the FDA, EMA, and Swiss Medic. These guidelines ensure that manufacturers adhere to specific practices to guarantee the consistent high quality of their products. Companies must comply with GMP regulations to obtain authorisation and licensing for manufactured goods.
To find out more about GMP here.
What is Continuous Chromatography?
Continuous chromatography, also known as Multi-column countercurrent solvent gradient purification (MSCGP), shares similarities with HPLC but stands out due to its fully automated nature, utilisation of multiple columns, and continuous loading process. This innovative method ensures high product yields while maintaining purity levels and typically reduces solvent consumption, promoting environmentally friendly chemistry practices.
To learn more, take a look at our video above or view our whitepaper.
Further topics of interest
CMC
Development
Click here >>
Analytical
Capabilities
Click here >>
Commercial
API
Click here >>
Quality &
Regulatory
Click here >>
Research Grade
Production
Click here >>
About
Bachem
Click here >>
Contact us