The development of GalNAc (N-acetylgalactosamine) oligonucleotide conjugates for the targeted delivery of RNA interference therapeutics to the liver enabled great advances in oligonucleotide potency. With the successful translation in human clinical trials, this strategy has been widely adopted for a variety of nucleic acid therapeutics, including antisense oligonucleotides (ASOs) and anti-microRNAs (anti-miRs). GalNAc conjugation enables oligonucleotides to overcome the human body’s defense system to keep invading RNAs from getting inside cells. This approach is applied to allow for the efficient delivery of oligonucleotide therapeutics including ASOs and small interfering RNAs (siRNAs) into targeted tissues and cells. As shown in Figure 1, several GalNAc oligonucleotide conjugates are known to be in clinical development with many more in discovery and preclinical development. In addition, GalNAc oligonucleotide conjugates including Givlaari® (givosiran), Oxlumo® (lumasiran), and Leqvio® (inclisiran) have gained approval from regulatory authorities.
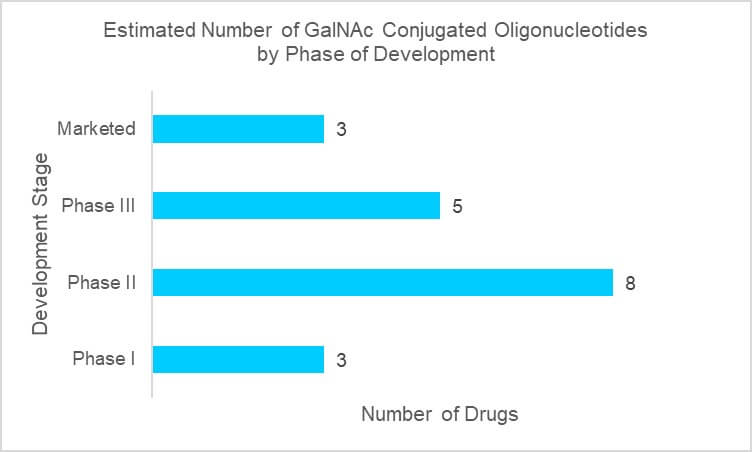
Figure 1 – GalNAc Conjugated Oligonucleotides in Clinical Phases (Source: GlobalData)
Approved GalNAc-siRNA Conjugates
Givlaari (givosiran), developed by Alnylam Pharmaceuticals, is approved in both the U.S. and Europe for the treatment of acute hepatic porphyria (AHP), a rare condition in which patients can experience potentially life-threatening abdominal pain, vomiting and seizures [1]. Givosiran targets the ALAS1 (aminolevulinate synthase 1) gene to treat AHP. The drug is a siRNA, covalently linked to a ligand containing three N-acetylgalactosamine (GalNAc) residues (Figure 2). Alnylam Pharmaceuticals is seeking to expand access to patients in additional regions and has submitted New Drug Applications with the regulatory authorities in Japan and Brazil where the drug is under priority review [2].
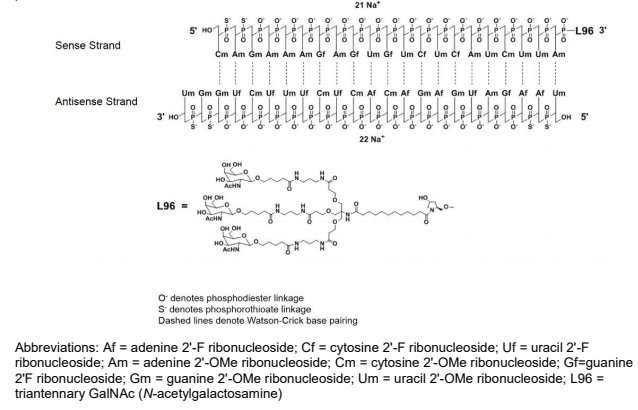
Figure 2 – Structure of givosiran (Source: U.S. FDA)
Alnylam Pharmaceuticals also developed Oxlumo (lumasiran) which the U.S. FDA and EMA recently approved in 2020. Oxlumo is a siRNA therapeutic targeting hydroxyacid oxidase 1 (HAO1). The drug is a siRNA covalently linked to a ligand containing N-acetylgalactosamine (GalNAc) (Figure 3). Oxlumo is indicated for the treatment of primary hyperoxaluria type 1 (PH1), a rare genetic disease, to lower the levels of oxalate in both adult and pediatric patients making it the first RNAi therapeutic approved in the U.S. for both children and adults [2].
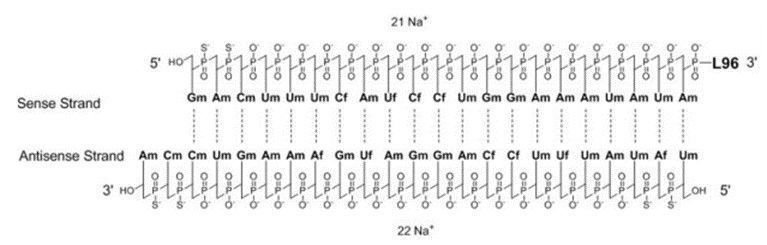
Figure 3 – Structure of lumasiran sodium (Source: U.S. FDA)
Leqvio (inclisiran) is a cholesterol-lowering siRNA conjugated to N-acetylgalactosamine (GalNAc) (Figure 4). The drug, under development by Novartis, received marketing authorization in the EU in December 2020 for use in adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet. Also in December, the U.S. FDA issued a complete response letter (CRL) concerning the new drug application (NDA) for inclisiran. Due to unresolved facility inspection-related conditions, the regulatory authority was not able to approve the NDA by the Prescription Drug User Fee Act (PDUFA) action date of December 23, 2020. Novartis plans to work with the FDA and their contract manufacturer to decide next steps and complete the review as quickly as possible [3].
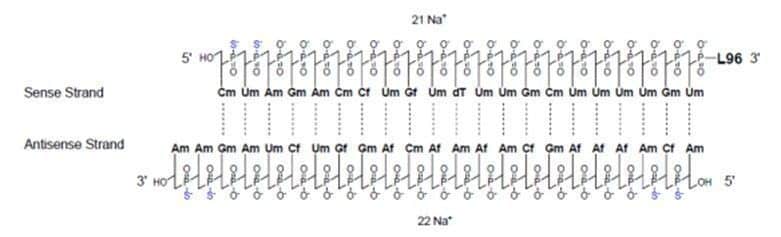
Figure 4 – Structure of inclisiran (Source: European Medicines Agency)
Conclusion
Recent drugs approvals have highlighted that delivery of oligonucleotides with GalNAc has reached clinical success. There is hope that these developments will encourage the advancement of further therapies for rare or untreatable diseases. At Bachem, we have a strong expertise in API manufacturing, and we support pharmaceutical companies in their development of new oligonucleotide modalities for new therapeutic applications. As a part of our service we offer the manufacture of oligonucleotide-based API. Our technological leadership and innovative strength have been the cornerstones of our success since the very beginning of our company. We look forward to working with our partners to bring medical advances and new oligonucleotide-based treatments to patients worldwide.
References
[1] FDA Approves Givosiran for Acute Hepatic Porphyria. U.S. Food and Drug Administration 2021.
[2] GlobalData 2021.
[3] Novartis Receives Complete Response Letter from U.S. FDA for Inclisiran. Novartis 2020.
Oligonucleotide NCEs
Emerging as a new treatment option in rare and orphan disease areas, oligonucleotides have matured into a drug class with a broad indication spectrum.
Subscribe to our newsletter
"*" indicates required fields
