The recent introduction of new drugs in the areas of diabetes, obesity, and cardiovascular disease have greatly increased the demand for manufacturing of polypeptides In turn, rapidly increasing commercialization of peptide drugs has driven an industrial intensification in peptide API manufacturing pushing to meet quality, efficiency, cost and volume criteria.
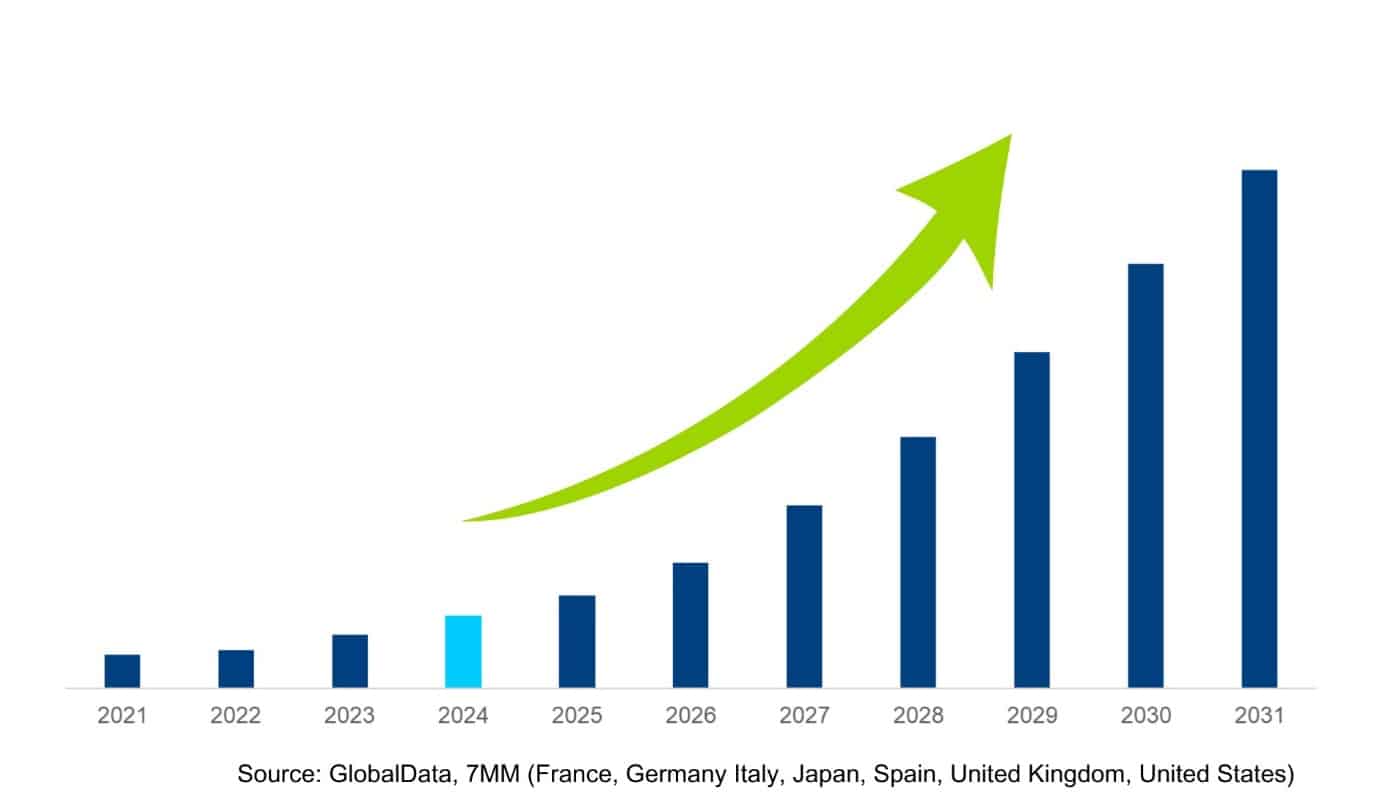
«Obesity Market across 7MM set to
surpass $37 billion in 2031»
For commercial-scale peptide API manufacturing, there are in principle two predominant production methodologies: synthetic or recombinant.
Comparison of synthetic vs. recombinant peptide synthesis
Both approaches offer some distinct advantages and face unique challenges, essential for stakeholders in the pharmaceutical industry to understand when selecting the most appropriate production strategy. Here we want to take a look at current state-of-the-art, strengths and weaknesses of each approach, and where the future is heading.
Recombinant Peptide Synthesis
Recombinant peptide synthesis is a biotechnological process that involves the production of peptides using genetically engineered organisms such as bacteria, yeast, or mammalian cells. In this method, the DNA sequence encoding the desired peptide is inserted into the genetic material of a host organism. This modified DNA, typically incorporated into a plasmid or other vector, instructs the host organism’s cellular machinery to produce the peptide as it grows and divides.
During recombinant synthesis, the host organism translates the recombinant DNA into the peptide chain, often with the capability to perform post-translational modifications, which are chemical changes made to the peptide after it has been synthesized. These modifications can include phosphorylation, glycosylation, and the formation of disulfide bridges, among others, which are crucial for the functionality of many biologically active peptides.
The peptides produced are then harvested from the culture medium or cell mass and purified through various biochemical techniques to remove any biological contaminants and unwanted byproducts. This method is particularly useful for producing large quantities of peptides and is favored for its ability to naturally incorporate complex peptide structures and modifications that are difficult to achieve through synthetic chemical methods.
Advantages in recombinant synthesis of peptides
- Cost-effective for large-scale production: Recombinant technology is more cost-effective at scale due to the lower cost of microbial or cell culture media compared to synthetic amino acids. This makes it suitable for the production of large volumes of peptides.
- Allows 3D protein folding and post-translational modifications: Recombinant synthesis excels in manufacturing peptides with complex tertiary structures and specific post-translational modifications, which can be crucial for biological activity and drug efficacy.
- Small waste footprint and more sustainable processes: Leveraging biological systems reduces chemical waste and energy consumption associated with synthetic processes, aligning with green manufacturing principles and reducing the ecological footprint. However, extensive purification needs might reduce overall benefits.
- Able to produce very long-chain peptides: Compared to synthetic methods like solid-phase peptide synthesis (SPPS), recombinant manufacturing’s use of biological organism and their ribosomal machinery makes polypeptide synthesis very efficient even at chain lengths including hundreds of amino acids.
The cost-effectiveness for large-scale production and the small waste footprint and sustainable processes will benefit from the increasing market demand.
Challenges in recombinant synthesis of peptides
- Complex purification needs and difficult impurity profiles: The presence of host cell proteins, nucleic acids, and other impurities necessitates extensive downstream processing, which can be both cost-intensive and technically challenging, reducing the overall process efficiency
- Potential for immunogenicity and biological contamination: The introduction of novel peptide sequences via recombinant methods may increase the risk of eliciting an immune response in patients, which could limit the clinical utility of the produced peptides
- Lengthy development cycles and up-front costs: The creation and optimization of genetic constructs, along with the scale-up of fermentation processes, require significant time investments, which can delay the initial production phase.
- Limited ability to accommodate modifications: Complex chemical modifications such as backbone stabilizations, artificial amino acids or unusual side chains can usually not be accommodated
With technological advancement, it is to be expected that challenges around lengthy development cycles and up-front costs, as well as the limited ability to accommodate modifications, will be smaller in the near future.
Synthetic Peptide Synthesis
Synthetic peptide synthesis refers to the chemical process used to construct peptides in a laboratory setting without the use of biological organisms. This method involves sequentially linking amino acids to form a peptide chain through a series of chemical reactions. The most common techniques used in synthetic peptide synthesis include solid-phase peptide synthesis (SPPS) and liquid-phase peptide synthesis (LPPS).

Large-scale SPPS reactor at Bachem
In SPPS, the peptide is assembled on a solid support, typically a resin, which facilitates the automation and purification of the synthesis process. Each amino acid is added sequentially to the growing peptide chain, with cycles of coupling (adding the amino acid) and deprotection (removing protective groups such as Fmoc or Boc) occurring until the desired sequence is complete. This method allows for precise control over the peptide’s sequence and composition, including the incorporation of non-natural amino acids and various modifications.
Synthetic peptide synthesis is favored for its ability to produce peptides quickly and with high purity. It is particularly effective for creating short to medium-length peptides and is widely used in research and pharmaceutical development for producing peptides used in therapeutics, diagnostics, and as research tools.
Advantages in synthetic synthesis of peptides
- Precision, customization, and chemical modifications: Synthetic peptide production enables the incorporation of diverse synthetic elements, including non-proteinogenic amino acids and various biochemical or biophysical probes. This method is particularly adept at incorporating post-translational modifications that enhance peptide functionality, stability, or bioavailability.
- Automation, scalability, and speed: Automated Solid-Phase Peptide Synthesis (SPPS) facilities the rapid production of peptides, significantly reducing time to market. This method’s scalability is advantageous for meeting the swift pace of demand in pharmaceutical applications.
- Elimination of biological contaminants and immunogenic triggers: Unlike recombinant methods, synthetic synthesis does not involve host organisms, thus eliminating the risk of contamination with biological agents such as viruses or prions, which simplifies the regulatory compliance process, particularly concerning BSE and TSE safety.
- High-quality peptides with known impurities: Chemical synthesis provides a high degree of control over the peptide’s composition and purity, minimal variability between batches and generally well-characterized impurities arising from predictable sources, such as incomplete coupling or deprotection reactions.
All of these advantages are becoming more and more important due to the increasing market demand.
Challenges in synthetic synthesis of peptides
- Complex starting materials and high material costs: Synthetic amino acids with protective groups are expensive building blocks, on top of specialized resins, reagents, and instruments.
- Scale-up and process optimization: Each increase in scale can introduce variations in mixing efficiency, temperature control, and the timing of reactions, which may affect the yield and purity of the final product. Addressing these scale-up challenges requires extensive process development and optimization, which can be time-consuming and costly, often necessitating iterative testing and modifications to achieve commercially viable production.
- High process mass intensity and organic solvent waste: Repetitive coupling, deprotection and washing steps consume resources and produce a lot of solvent waste. Synthesis and purification have the highest impact on process-mass-intensity, a measure for sustainability. Efforts to mitigate these impacts require advanced waste management and solvent recovery systems, which can further increase operational complexity and cost.
- Error accumulation is prohibitive for longer peptides: Synthesizing peptides longer than 50 amino acids poses numerous challenges, including lower reaction yields and higher probabilities of sequence errors or misfolding. These issues stem from the accumulation of minor errors in each synthesis step, which are magnified as the peptide lengthens.
Technical innovations will reduce the downsides of high material costs, scale-up and process optimization and high process mass intensity & organic solvent waste.
Chemical synthesis and hybrid approaches gain prominence for next generation peptide APIs
The current success of emerging peptide drugs for various indications is mostly owed due to solving pharmacokinetic challenges such as peptide stability, bioavailability, half-life, efficacy, renal clearance, and low immunogenicity by chemical modifications of backbone, non-proteinogenic amino acids, or side chains.
We also observe that growing molecule complexity, rising demand for speed and technological innovations drive chemical synthesis advantage and make this the preferred choice for next-generation drugs. Chemical synthesis excels in producing stable molecules, aligning with the trend towards oral administration as well. The faster production offered by chemical synthesis shortens the time to clinic and market, aligning with the industry’s push for accelerated drug development. Technological innovations such as soluble-anchor based Molecular HivingTM for synthesis and MCSGP for continuous purification support the scalability of commercial production, making chemical synthesis an ever-more attractive option for pharmaceutical companies seeking to stay at the forefront of innovation.
That being said, choosing between synthetic and recombinant peptide synthesis methods still requires a balanced consideration of several factors, including the specific peptide’s complexity, production volume, cost constraints, and the desired timeline for development. The future of peptide drug development will likely see innovations that address the current limitations of both recombinant and synthetic methods, ensuring that the pharmaceutical industry can meet the growing demand for peptide-based therapeutics with efficiency and agility.
Additionally, new hybrid or semi-synthetic approaches with partially recombinant, partially synthetically produced (or modified) peptide fragments which get ligated or conjugated into the full-length complex API offer an attractive and welcome expansion of the ever-growing repertoire of drug manufacturers.
«The single or combined use of chemical and biological recombination synthetic approaches allows the efficient and reliable production of synthetic peptides on large scales. These peptides can be further modified in a site-specific manner through chemical synthesis or genetic code expansion to enhance their stability and physiological activity.»
So, while chemical expertise and synthetic methods seem to have the market edge in the coming years, the final story is – as always – not yet written.
One thing can however already be concluded: These exciting developments within a fruitful innovation and competition space ultimately come to the benefit of patients.
References:
Subscribe to our general newsletter
"*" indicates required fields
