MEET US AT Alzheimer’s Association International Conference

We invite you to meet us at the Alzheimer’s Association International Conference (AAIC) 2017. The meeting will take place at the ExCeL London in London, United Kingdom from July 16 to 20, 2017.
AAIC brings together the world’s leading professionals involved in dementia care and neuroscience research to learn and share their latest advancements and results in this field of science.
Bachem supports its customers in the pursuit of groundbreaking discoveries that further scientific advances, particularly in the field of medicine. Bachem’s offer for Alzheimer’s research comprises a broad choice of amyloid peptide fragments including Tau fragments and Aβ mutant peptides. A comprehensive catalog of biochemicals deliverable ex-stock, an exclusive custom syntheses service for research labs and a full range of services to the pharma and biotech industries complete our service portfolio.
We are excited to meet with our customers, learn their needs for peptides and discuss how Bachem can help to advance their research.
We invite you to visit us at our Booth #108:please contact us to schedule a meeting in advance.
We look forward to meeting you at AAIC 2017!
SECRETASES
A key step in the pathogenesis of AD is the proteolysis of amyloid precursor protein (APP) resulting in the formation of amyloid β-peptides. APP is expressed ubiquitously in a number of isoforms consisting of 695 to 770 amino acids. Its degradation products Aβ40 and Aβ42 are the principal components of the cerebral plaques found in the brains of patients with AD. APPs are integral membrane proteins with a single membrane-spanning domain, a large extracellular glycosylated amino-terminus and a shorter cytoplasmic carboxy-terminus. The amyloid β-peptide is located at the cell surface, with part of the peptide embedded in the membrane. Processing of APP involves ectodomain shedding by either α-secretase (non-amyloidogenic pathway) or β-secretase (BACE1; amyloidogenic pathway) and subsequent cleavage by γ-secretase (see Fig. 1a and b). Cleavage of APP by BACE1 generates a soluble 100 kD amino-terminal fragment (sAPPβ) and a 99-amino acid fragment (C99 or CTFβ, ~12 kD) inserted in the membrane (amyloidogenic pathway, Fig. 1a). α-Secretase (TACE, TNF-converting enzyme) cleaves APP yielding the neurotrophic and neuroprotective fragment sAPPα and an 83-residue carboxy-terminal fragment (C83 or CTFα) (non-amyloidogenic pathway, Fig. 1b). Action of γ-secretase releases the APP intracellular domain (AICD) together with amyloid β-peptides from CTFβ or P3 peptide from CTFα. Additional proteolytic sites between Leu420 (Aβ 49) and Val421 (ε-cleavage site) and Val417 (Aβ46) and Ile418 (ζ-cleavage site) have been observed. The resulting fragments are further degraded yielding Aβ40/42 or P3. The non-amyloidogenic pathway could be promoted by neuronal activity, as the production of sAPPα increases in response to electrical activity and activation of muscarinic acetylcholine receptors.
Amyloid Precursor Protein (661-730) IKTEEISEVK MDAEFRHDSG YEVHHQKLVF FAEDVGSNKG AIIGLMVGGV VIATVIVITL VMLKKKQYTS (Aβ42 marked red, cleavage site of α-secretase/neprilysin-2 marked blue)
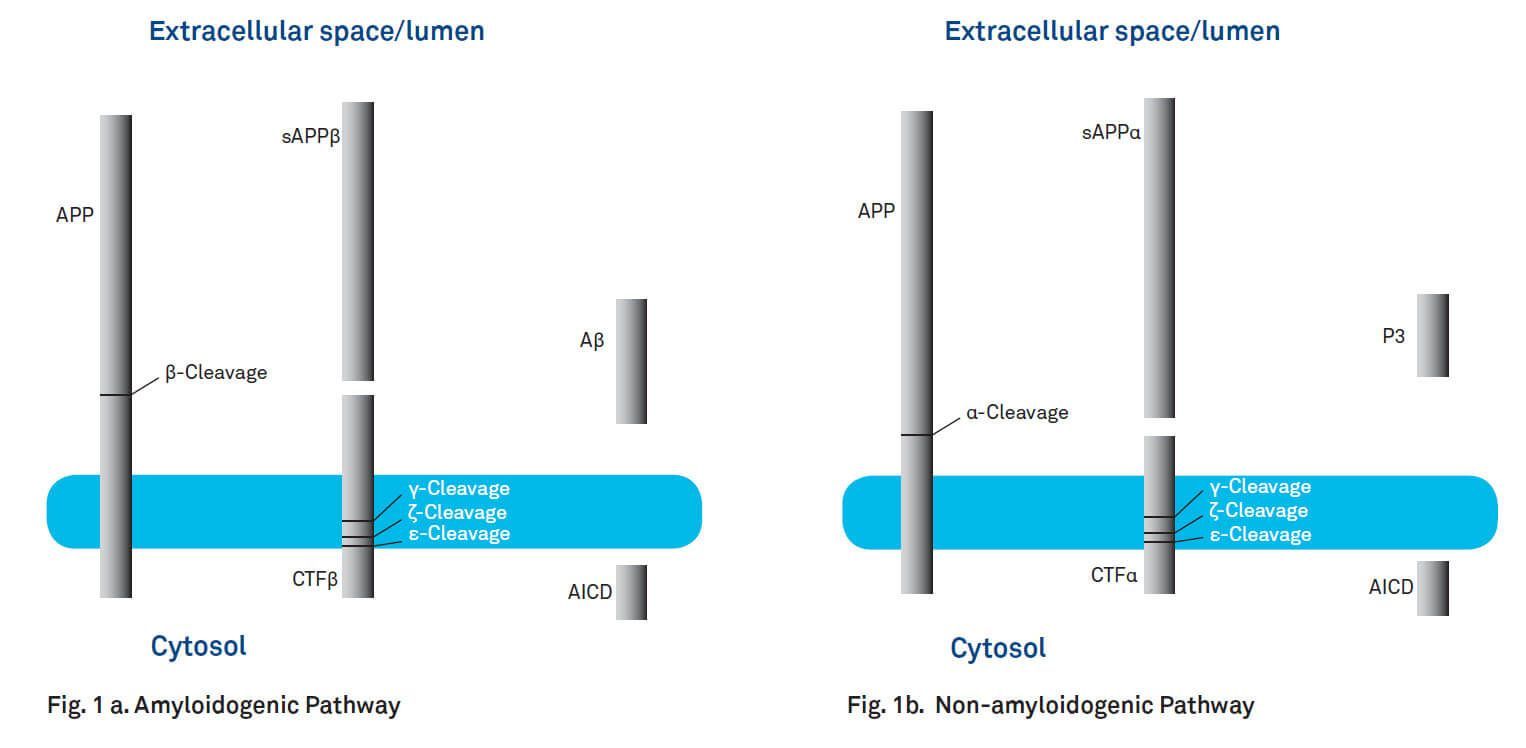
Fig. 1. Processing of amyloid precursor protein. a. Amyloidogenic Pathway. Cleavage of APP by β-secretase (BACE1) and γ-secretase leads to the generation of Aβ. b. Non-amyloidogenic Pathway. Processing of APP by α-secretase and γ-secretase yields P3.
Aβ40 is the major type of amyloid β-peptides secreted into normal human cerebrospinal fluid. Though the longer and more hydrophobic Aβ42 is produced in much smaller amounts, it is the major Aβ species found in cerebral plaques due to its high propensity for aggregation. APP mutations near the β- and γ-secretase cleavage sites may enhance or reduce formation of Aβ42. The Swedish double mutation (K670N, M671L) near the β-secretase cleavage site causes early-onset Alzheimer’s disease as it facilitates proteolysis by BACE1, whereas the Icelandic mutation (A673T, Aβ A2T) protects from AD as it impedes the attack of the enzyme. Early-onset AD is the consequence of the Iranian APP mutation T714A (Aβ T43A) situated close to the cleavage site of γ-secretase, and of mutations such as K687N (Aβ K16N) which impede non-amyloidogenic APP processing by α-secretase. β- and γ-secretase are main targets for developing AD therapies as inhibiting BACE or TACE could reduce the burden of amyloid β-peptide, and thus, plaque formation in AD patients‘ brains. Promoting the non-amyloidogenic variant of APP degradation by activation of α-secretase has also shown therapeutic potential.
α-Secretase
As α-secretase cleaves membrane-bound APP in the middle of the amyloid region, Aβ peptides cannot be generated. α-Secretase belongs to the ADAM (a disintegrin and metalloprotease) family of proteases, membrane-anchored proteins depending on zinc. ADAMs play a role in diverse biological processes such as fertilization, neurogenesis, and the activation of growth factors and immune regulators. Three candidates of this proteinase family can process APP: ADAM9, ADAM10 and ADAM17 (TACE). All of them contain an autoinhibitory domain that has to be removed for activity, a proteolytic domain, a disintegrin domain, a cysteine-rich domain, and, most important for APP processing, a transmembrane domain. ADAMs may contain a zinc-binding motif, HEXXH, in the catalytic domain. To investigate α-secretase activity, human ADAM9, ADAM10, or ADAM17 were cloned and expressed in COS-7 cells. The endogenous APP was cleaved by each of the three enzymes. In addition, TACE cleaves pro-TNF-α, releasing the extracellular domain (TNF-α) in a manner similar to that of APP. ADAM10 is expressed in mouse and human brain, cleaves APP-derived peptides at the main α-secretase cleavage site between position 16 and 17 of the Aβ region, and shows α-secretase activity in cultured cells. ADAM10-deficient mice have been generated, but their early lethality prevented a reliable analysis of ADAM10 function in vivo, especially in neuronal cells. A study aiming at the tissue distribution of ADAM9, ADAM10, and ADAM17 showed that the mRNA of ADAM9 is ubiquitously expressed in human tissues, whereas ADAM10 mRNA is only observed in kidney, spleen, lymph node, thymus, liver, bone marrow, and brain. Strong expression of ADAM17 mRNA is found in macrophages. In human brain, ADAM9 mRNA expression is higher than the expression of ADAM10 and ADAM17. Recent results indicate that ADAM10 could be the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. ADAM10 was shown to prevent plaque formation and hippocampal deficits in an AD mouse model already in 2004. The neuropeptides PACAP-27 and PACAP-38 act as natural activators of the enzyme. Development of small molecule activators or stimulation of α-secretase (ADAM10/17) expression could be an alternative to β- and γ-secretase inhibition in the management of AD. Notably, levels of melatonin, an endogenous promoter of ADAM10/17 expression, are decreased in AD patients. Etazolate (EHT-0202), a selective GABA-A receptor modulator, the PKC modulator bryostatin, and the polyphenol epigallocatechin gallate from green tea are evaluated in clinical trials. These compounds stimulate α-secretase by different mechanisms. Neprilysin-2, another membrane-anchored metalloprotease, probably is the major enzyme involved in cerebral Aβ clearance. The protease cleaves Aβ between Lys16 and Leu17, which corresponds to the α-secretase cleavage site.
β-Secretases
β-secretase (BACE1) belongs to the pepsin/renin family of aspartic proteases. BACE1 and its homolog BACE2 constitute a new branch of this family. BACE1 is activated by a furin-like protease which removes the propeptide masking the active site. BACE is a type 1 transmembrane protease, containing a single transmembrane domain near the carboxy-terminus, an N-terminal signal sequence including a propeptide region, and two aspartates in its ectodomain (Asp93 and Asp289). These residues are essential for enzymatic activity. In addition to cleaving APP at the β-secretase site (Leu671-Asp672), BACE cuts APP further downstream within the amyloid region, between Tyr10 and Glu11 of Aβ. Glu cyclizes yielding (Pyr11)-Aβ (11-40/42). Both truncated Aβ peptides form stable aggregates, they are constituents of cerebral plaques. BACE1 also cleaves the APP-like proteins APLP1 and APLP2 (both are important modulators of glucose and insulin homeostasis) and further membrane-associated proteins. Human β-secretase is expressed in many cell types, the highest concentration of the enzyme is detected in the neurons of the brain. The gene for BACE1 is located on chromosome 11, the homolog BACE2 maps to chromosome 21, raising the possibility that BACE2 contributes to AD associated with Down syndrome. Down syndrome patients secrete more Aβ from birth onwards and develop AD by age 50. BACE2 cleaves APP in a β-secretase-like manner. It is strongly expressed in heart, kidney, placenta, and, only in low amounts, in brain. So, BACE2 plays a minor, if any, role in the formation of cerebral plaques. Merely a lack of BACE1 results in an almost complete block of Aβ generation in neurons. Comparison of BACE with other aspartic proteases such as cathepsin D and E, napsin A, pepsin, and renin revealed little similarity in substrate preference or inhibitor profile. The active site of β-secretase is more accessible than that of pepsin; the S2 and S4 subsites are relatively hydrophilic and open to solvent. These differences could be exploited for the design of selective inhibitors. Several β-secretase inhibitors containing a moiety that mimics the transition state formed during aspartic protease catalysis, have been developed (Fig. 2 shows an example). These peptide analogs are based on the APP motif EVNLDAEF cleaved by BACE between leucine and aspartic acid.
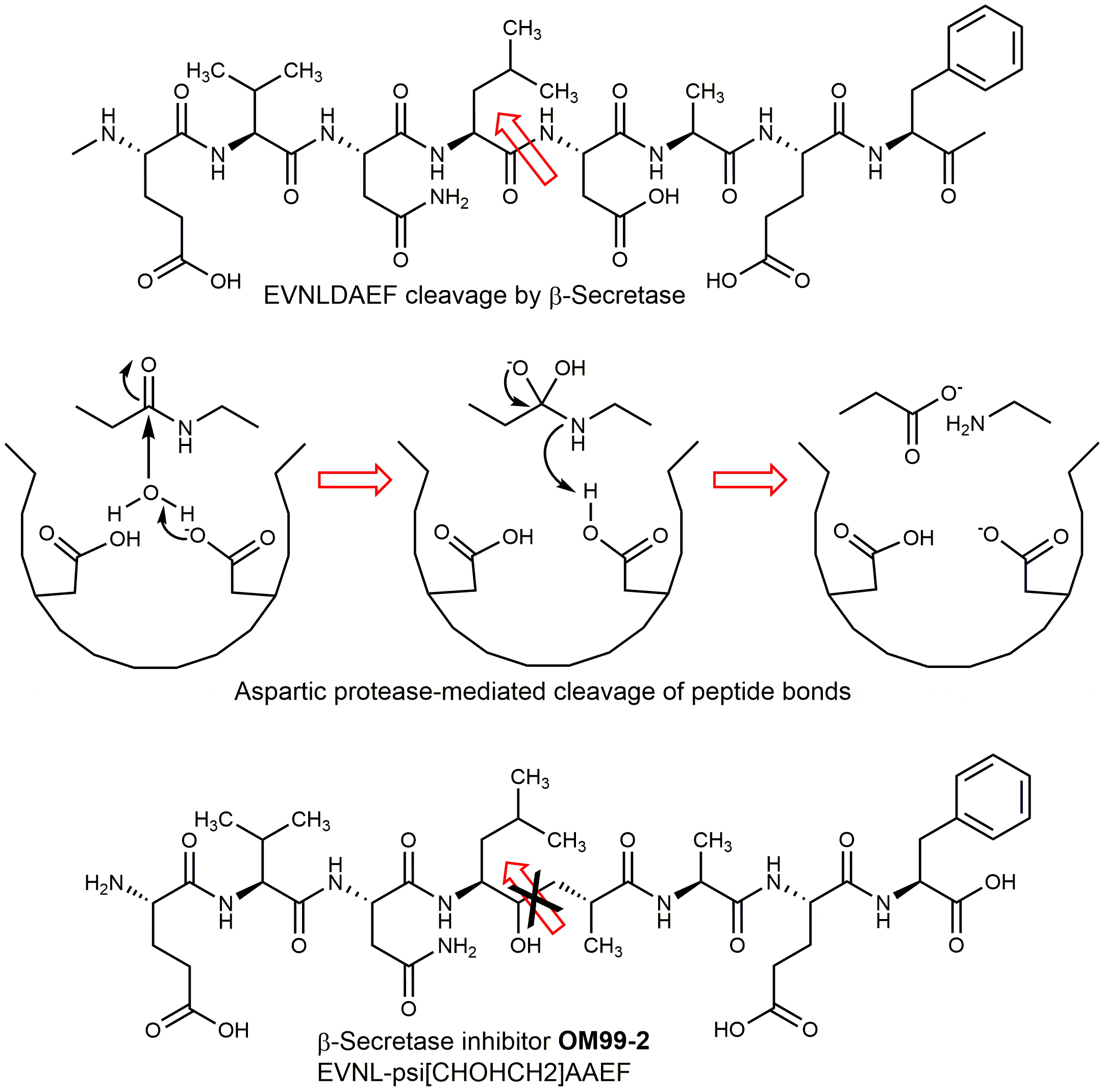
Fig. 2. Development of β-secretase inhibitors. The Ile-Asp peptide bond is replaced by a hydroxyethyl moiety mimicking a transition state of the enzyme-substrate complex. Whereas the degradation products of the peptide are released after cleavage, the hydroxyethyl analog cannot be processed and remains bound to the active site.
β-Secretase could be an optimal therapeutic target for the prevention and treatment of AD, since BACE1-deficient mice show reduced Aβ production. The enzyme belongs to an intensively studied type of proteases. Therapeutically useful inhibitors have been developed for other aspartic proteases such as renin or HIV protease. The crystal structure of BACE1 is known, which facilitates the rational design of new inhibitors. On the other hand, BACE1 was shown to modulate myelination in the central and peripheral nervous system, which raised some concern about the use of BACE1 inhibitors in the treatment of AD. Currently, several small-molecule β-secretase inhibitors are in clinical evaluation. Verubecestat (MK-8931), initially developed for the management of mild to moderate AD, has moved to phase IIII, as did the BACE1 inhibitor lanabecestat (AZD-3293, LY3314814) due to encouraging results in mild AD cases. Clinical studies of the brain-penetrant inhibitor JNJ- 54861911 showed a marked and long-lasting decrease of Aβ40 levels in plasma and cerebrospinal fluid. CNP-520 and E-2609 are further small-molecule BACE1 inhibitors having reached phase II/III. Moreover, the antidepressant levomilnacipran could find use in AD therapy, as it showed a remarkable BACE1 inhibitory activity.
γ-Secretase
γ-Secretase is a unique membrane-bound protease responsible for the intramembrane cleavage of a subset of type l membrane-spanning proteins including APP and Notch. γ-Secretase plays an important role in the pathogenesis of AD by releasing the carboxy-terminus of Aβ, generating Aβ40 and the more amyloidogenic Aβ42. γ-Secretase cleaves the hydrophobic integral membrane domain of its substrates, resulting in the release of protein fragments at the luminal (extracellular) and at the cytoplasmic side of the membrane. This cleavage represents an example of regulated intramembrane proteolysis (RIP). γ-Secretase is a multiprotein complex consisting of at least four proteins: presenilin, nicastrin, anterior pharynx (APH-1), and presenilin enhancer 2 (PEN-2). All of them are required for full proteolytic activity. The presenilins (~50 kD) are polytopic transmembrane proteins with nine putative transmembrane domains and appear to provide the active core of this protease. Two mammalian homologs, PS1 and PS2, exist. The presenilins undergo autocatalytic proteolysis to generate amino-terminal and carboxy-terminal fragments, which remain associated as functional heterodimer complexes. These contain nicastrin and other molecules that are important for γ-secretase activity. Nicastrin, a glycosylated ~130 kD integral membrane protein, binds to both N-terminal and C-terminal fragment of presenilin. Nicastrin requires presenilin to leave the endoplasmic reticulum and to reach the cell surface. In presenilin-deficient cells, the nicastrin receptor accumulates in the endoplasmic reticulum. Nicastrin is supposed to be one of the stabilizing factors of the presenilin fragments. Additionally, APH-1, a ~30 kD multimembrane protein, is needed for the correct subcellular transport of nicastrin to the cell surface. PEN-2, a small, hairpin-like membrane protein with a molecular weight of ~12 kD, seems to be required for the cleavage of presenilin when it forms the multiprotein complex with APH-1 and nicastrin. Apparently, all four proteins exert regulatory effects on each other. γ-Secretase requires the aspartic protease activity of presenilin-1. Two aspartate residues (Asp257 and Asp385) located in the transmembrane domains 6 and 7 are essential for catalytic activity (cf. Fig. 2). γ-Secretase could be considered an aspartic protease, though the exact structure of the active site still is unknown. The molecular weight of this complex is unknown, estimates vary from 250 – 1000 kD. Apart from APP, γ-secretase cleaves various substrates including Notch 1-4 and the Notch ligands Delta and Jagged. Notch is a cell surface receptor, which, when activated by ligands such as Jagged and Delta, is cleaved in the membrane liberating an intracellular domain of Notch. γ-Secretase-mediated Notch-signaling plays an essential role in the regulation of cell fate during the development of many organ systems including the brain as indicated by embryonic lethality and defective neurogenesis that is identical in Notch-1 and presenilin-1 deficient mice. Further studies are still needed to better understand the molecular function of the different subunits. In addition, the subcellular compartments in which the different subunits bind to each other, remain to be determined. Inhibition of γ-secretase may be a therapeutic approach for limiting the generation of the amyloidogenic Aβ42. γ-Secretase inhibitors have to block the proteolysis of APP with high selectivity, so that Notch, its major physiological substrate, and other proteins still can be processed by the enzyme. Certain non-steroidal anti-inflammatory drugs (NSAIDs) and other small organic molecules modulate γ-secretase and selectively reduce Aβ42 levels without affecting Notch cleavage. Novel therapeutic approaches for reducing Aβ production in AD patients could be derived from the observation that ATP binds to γ-secretase and selectively activates APP processing.
Conclusion
Treating Alzheimer’s disease remains one of the greatest challenges in neurology. Current drugs, such as the acetylcholinesterase inhibitors tacrine, donezepil, galantamine, and rivastigmine as well as the NMDA receptor antagonist memantine only improve symptoms, but do not show profound disease-modifying effects. Inhibition of secretase activity, inhibition of amyloid-β aggregation, and immunotherapy are relevant therapeutic approaches in current AD drug development. Specific γ-secretase inhibitors have been developed, but their use in humans may be accompanied by side effects, as the processing of Notch and other protein substrates by the enzyme could be inhibited concomitantly. Studies in BACE1-deficient mice showed that BACE1 inhibitors could reduce Aβ production. To date several drugs are in clinical trial. α-Secretase activators promoting non-amyloidogenic APP degradation have also gained interest. In parallel to efficient disease-modifying therapy, effective ways of defining genetic predisposition and methods of early diagnosis, e.g. via biomarkers, are required.
References
L. Hong et al., Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 290, 150-3 (2000) 11021803
D.R. Howlett et al., In search of an enzyme: the beta-secretase of Alzheimer‘s disease is an aspartic proteinase. Trends Neurosci. 23, 565-70 (2000)
P. Pasalar et al., An Iranian family with Alzheimer’s disease caused by a novel APP mutation (Thr714Ala). Neurology 58, 1574-5 (2002) 12034808
M. Asai et al., Putative function of ADAM9, ADAM10, and ADAM17 as APP alpha-secretase. Biochem. Biophys. Res. Commun. 301, 231-5 (2003)
B. De Strooper, Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron 38, 9-12 (2003)
D.J. Selkoe and D. Schenk, Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003; 43, 545-84 (2003) 12415125
S.F. Lichtenthaler and C. Haass, Amyloid at the cutting edge: activation of alpha-secretase prevents amyloidogenesis in an Alzheimer disease mouse model. J. Clin. Invest. 113, 1384-7 (2004)
X. Hu et al., Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520-25 (2006)
E. Kojro et al., The neuropeptide PACAP promotes the alpha-secretase pathway for processing the Alzheimer amyloid precursor protein. FASEB J. 20, 512-514 (2006)
D. Spasic et al., Presenilin-1 maintains a nine transmembrane topology throughout the secretory pathway. J. Biol. Chem. 281, 26569-77 (2006)
J. Tan et al., Residues at P2-P1 positions of epsilon- and zeta-cleavage sites are important in formation of beta-amyloid peptide. Neurobiol. Dis. 36, 453-60 (2009) PMC3520095
H. Yamakawa et al., beta-Secretase inhibitor potency is decreased by aberrant beta-cleavage location of the “Swedish mutant” amyloid precursor protein. J. Biol. Chem. 285, 1634-42 (2010) PMC2804321
P. Kaden et al., Novel APP/Aβ mutation K16N produces highly toxic heteromeric Aβ oligomers. EMBO Mol. Med. 4, 647-59 (2012) PMC3407951
N. Jurisch-Yaksi et al., A fast growing spectrum of biological functions of γ-secretase in development and disease. Biochim. Biophys. Acta. 1828, 2815-27 (2013)
R.A. Marr and D.M. Hafez, Amyloid-beta and Alzheimer’s disease: the role of neprilysin-2 in amyloid-beta clearance. Front. Aging Neurosci. 6, 187 (2014) PMC4131500
A. Kokawa et al., The A673T mutation in the amyloid precursor protein reduces the production of beta-amyloid protein from its beta-carboxyl terminal fragment in cells. Acta Neuropathol. Commun. 3, 66 (2015) PMC4632685
R. MacLeod et al., The role and therapeutic targeting of α-, β- and γ-secretase in Alzheimer’s disease. Future Sci. OA, 1 (2015) DOI 10.4155/fso.15.9
P. Saftig and S.F. Lichtenthaler, The alpha secretase ADAM10: A metalloprotease with multiple functions in the brain. Prog. Neurobiol. 135, 1-20 (2015)
M. Qian et al., The Distinct Role of ADAM17 in APP Proteolysis and Microglial Activation Related to Alzheimer’s Disease. Cell. Mol. Neurobiol. 36, 471-82 (2016)
Y. Tan et al., Anti-Alzheimer Therapeutic Drugs Targeting γ-Secretase. Curr. Top. Med. Chem. 16, 549-57 (2016)
H.M. Wilkins and R.H. Swerdlow, Amyloid precursor protein processing and bioenergetics. Brain Res. Bull. 2016 doi:10.1016/j.brainresbull.2016.08.009 PMC5316384
R. Yan, Stepping closer to treating Alzheimer’s disease patients with BACE1 inhibitor drugs. Transl. Neurodegener. 5, 13 (2016)
SECRETASE INHIBITORS AND MODULATORS IN CLINICAL DEVELOPMENT
Alzheimer’s disease (AD) research has focused on the amyloid cascade hypothesis which proposes that AD is caused by changes in amyloid beta (Aβ) stability and aggregation or changes in amyloid precursor protein (APP) expression. Blocking Aβ production by inhibiting the proteases required for its generation may serve to halt AD progression or prevent the disease (1). APP is cleaved by α-secretase or β-secretase and then further processed by γ-secretase. The sequential cleavage of APP by β-secretase and γ-secretase generates Aβ. The proposal of over-activated β- and γ-secretases or decreased α-secretase processing with aging has led to the design and development of secretase inhibitors and modulators (2).
Several secretase inhibitors and modulators have progressed to clinical development as shown in Table 1.
Table 1:Secretase Inhibitors in Phase I-III Clinical Development (3)
| Product Name | Active Ingredient | Condition Treated | Highest Phase | Companies |
|---|---|---|---|---|
| AZD3293 | lanabecestat | Alzheimer's Disease(III) | Phase III | Astex Pharmaceuticals, Eli Lilly and Company, AstraZeneca, Otsuka Pharmaceutical Co Ltd |
| JNJ-54861911 | -- | Alzheimer's Disease(III) | Phase III | Shionogi & Co Ltd, Janssen Pharmaceuticals Inc, Johnson & Johnson |
| E2609 | -- | Alzheimer's Disease(III) | Phase III | Eisai Co Ltd, Biogen, Eisai Limited, Eisai Inc |
| MK8931 | verubecestat | Alzheimer's Disease(III) | Phase IIII | Schering-Plough Corp, Pharmacopeia, Inc., Ligand Pharmaceuticals Inc, MSD KK, Merck & Co Inc |
| Cerebrate | pinitol | Alzheimer's Disease(II) | Phase II | Humanetics Corporation |
| CSP1103 | -- | Alzheimer's Disease(II) | Phase II | Chiesi Farmaceutici SpA, CereSpir Incorporated |
| LY3202626 | -- | Alzheimer's Disease(II) | Phase II | Eli Lilly and Company |
| PF03084014 | -- | Alzheimer's Disease(I), Oncology(I) | Phase II | Pfizer Inc |
| T3D959 | -- | Alzheimer's Disease(II) | Phase II | Bayer Ag, T3D Therapeutics, Midatech Pharma US Inc |
| NGP555 | -- | Alzheimer's Disease(I) | Phase I | NeuroGenetic Pharmaceuticals Inc |
| PF06648671 | -- | PAlzheimer's Disease(I) | Phase I | Pfizer Inc |
Phase III Candidates
Astra Zeneca and Eli Lilly are developing AZD3293, a β-secretase inhibitor. In 2016, The U.S. Food and Drug Administration (FDA) gave Fast Track designation to AZD3293 for AD. In 2017, Eli Lilly initiated a Phase III study of AZD3293 in early AD (3).
Janssen is developing a β-secretase inhibitor licensed from Shionogi known as JNJ54861911. In 2015, Janssen started a Phase II/III study in asymptomatic people at risk of developing Alzheimer’s dementia (4).
E2609 is a β-secretase inhibitor in Eisai’s pipeline. In 2016, Eisai reported that the U.S. FDA granted Fast Track designation for the development of E2609. Eisai and Biogen have initiated a Phase III clinical study to evaluate the efficacy and safety of E2609 in early Alzheimer’s disease (3).
MK8931 (verubecestat) is a β-secretase inhibitor that is being developed by Merck & Co. MK8931 is currently in Phase III clinical trials for the treatment of prodromal or the very early form of AD. In 2017, Merck reported that it completed enrollment in a Phase III study evaluating the safety and efficacy of MK8931 is patients with prodromal AD (3).
Phase II Candidates
Cerebrate (pinitol) is a γ-secretase inhibitor that is being developed by Humanetics Corporation. Cerebrate prevents the formation of Aβ plaques through selective inhibition of γ-secretase activity targeting APP. It is being developed for the treatment of mild to moderate AD. In 2016, Humanetics completed a Phase II trial of Cerebrate in patients with AD (3).
Chiesi Farmaceutici is developing CSP1103, a γ-secretase modulator that selectively reduces pro-inflammatory activities of microglial cells while increasing their ability to remove aggregated Aβ in the brain (3). In 2015, CereSpir reported that the company submitted a Special Protocol Assessment request to the U.S. FDA to initiate study design discussions for a Phase III clinical study of CSP-1103 to test its ability to slow the progression of Mild Cognitive Impairment (MCI) due to AD (3).
Eli Lilly is developing a β-secretase inhibitor known as LY3202626. In 2016, Eli Lilly initiated a Phase II study to evaluate the safety and the effect of the product on brain tau in participants with mild AD (3).
Pfizer is developing a γ-secretase inhibitor known as PF03084014. This small molecule is being developed for the treatment of AD and cancer. Although the company’s development programs for breast cancer and pancreatic cancer were terminated, the company has changed their strategy and reinitiated a Phase I study of PF-03084014 in patients with advanced solid tumor malignancy and T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (3).
T3D959 is a β-secretase inhibitor that is being developed for the treatment of AD. In 2016, T3D Therapeutics presented preliminary data from a Phase IIa study with T3D959 in patients with mild to moderate AD. Results from the study showed potential improvement in cognition, potential to improve motor function and penetration of the drug into the brain (5).
Phase I Candidates
NGP555 is a modulator of the γ-secretase complex that is being developed by NeuroGenetic Pharmaceuticals for the treatment and prevention of AD. NGP555 crosses the blood-brain barrier and is effective in lowering Aβ-42 and Aβ-40 while showing an increase in the Aβ-38 peptide. In 2017, the company completed a Phase I study evaluating the safety, tolerability and pharmacokinetics of NGP 555 in healthy subjects (3).
Pfizer is also developing a γ-secretase modulator known as PF06648671. In 2016, Pfizer completed a Phase I study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of repeat doses of PF-06648671 in healthy subjects (3).
Conclusion
β- and γ-secretase continue to be prime drug discovery targets for AD. At shop.bachem.com, Bachem offers over 250 different research peptides including Aβ peptides, β- and γ- secretase inhibitors, APP fragments and more for researchers and organizations studying AD. In addition, a comprehensive custom peptide synthesis service is provided and we offer the production of new chemical entities.
References
(1) K.W. Menting and J.A.H.F. Claassen, β-secretase inhibitor; a promising novel therapeutic drug in Alzheimer’s disease, Front Aging Neurosci. 165(6), (2014)
(2) J. Mendolia-Precoma et al., Therapies for prevention and treatment of Alzheimer’s disease, Biomed Res Int. 2589276, (2016)
(3) Medtrack (2017)
(4) Therapeutics, Alzforum (2017)
(5) Pipeline, T3D Therapeutics (2017)
MEET BACHEM: JANA WROBEL
Project Manager
How long have you been with Bachem? Where did you work before Bachem?
I am with Bachem since exactly one year now – my first working day was on June 6, 2016. I remember that day very well. After finishing my studies, I first worked at Merz Pharma in Frankfurt in the ADME/DMPK lab as a scientist and CRO manager and for the last three years I switched to the Strategic Sourcing department as a Junior Buyer, being responsible for the sourcing of packaging materials, CRO services (pre-clinical and clinical) and logistics.
Briefly, what do you do at Bachem?
I am involved in various API projects.
What is your academic background/degrees or training?
B. Eng. in Bioprocess Engineering
M. Sc. in Biotechnology/ Bio-Systems Technology
What do you like to do outside of work (interests, hobbies)?
I love to explore the world – either by traveling, hiking or biking. Since I´m living in Basel, I give free rein to my enthusiasm for the mountains. Whenever there is free time during the weekends, I try to get as close as possible to the mountains. Besides that, I (try to) go to the gym regularly, like to hang out with friends (e.g. at the Rhine river) and just enjoy my life (of course good food and drinks play quite an important role too).
What makes a perfect day for you?
Not knowing what the day will bring, when I wake up and going to bed with a happy smile in my face – that must have been a perfect day. And I can think of so many different things that could have contributed to this feeling!
What is your business motto?
Stay inspired and empowered by connecting with others and following your passion.
What do you like most about your job?
As a Project Manager, you are always learning – no two projects are alike. I like that a project manager’s world is ever changing, growing and evolving. And in this world, you get to interact with all these interesting and smart people who bring different perspectives and experiences to the project. And if you do it right, the collaborative effort can lead to the creation of something better than what you could produce by yourself. I like having a lot of responsibilities, face all sorts of pressure, have to work with a wide variety of stakeholders and customers, and a hundred other worries to resolve.
Have you had any particular expectation when you came to Bachem and have these been fulfilled?
As you spend so many hours per day at work, I think not only the work itself, but the people you work with contribute most to an enjoyable job. From the first day on, I felt very welcomed at Bachem. I appreciate the high interest to share knowledge and to develop together throughout the whole company. This professional level is accompanied by a very friendly, amicable and open atmosphere. …. So yes – expectations are fulfilled.
What is your preferred peptide?
Glucagon – this product is always good for a surprise.
Thank you very much Jana.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Probe of Alzheimer’s follows paths of infection-Harvard Gazette
Chemists synthesize molecular pretzels-Science Daily
Scientists discover GLP-1-like compound of benefit for diabetes research-Diabetes.co.uk
Frog slime kills flu virus-Emory University
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
M. Pigoni et al.
Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons.
Mol. Neurodegener. 11, 67 (2016)
F. Qu et al.
Dual Signal Amplification Electrochemical Biosensor for Monitoring the Activity and Inhibition of the Alzheimer’s Related Protease beta-Secretase.
Anal. Chem. 88, 10559-10565 (2016)
K. Ito et al.
Memantine reduces the production of amyloid-beta peptides through modulation of amyloid precursor protein trafficking.
Eur. J. Pharmacol. 798, 16-25 (2017)
H. Keranen et al.
Acylguanidine Beta Secretase 1 Inhibitors: A Combined Experimental and Free Energy Perturbation Study.
J. Chem. Theory Comput. 13, 1439-1453 (2017)
A.F Lopes Vilela and C.L.Cardoso
An on-flow assay for screening of β-secretase ligands by immobilised capillary reactor-mass spectrometry.
Anal. Methods 9, 2189-2196 (2017)
F.J.R. Rombouts et al.
Fragment Binding to β-Secretase 1 without Catalytic Aspartate Interactions Identified via Orthogonal Screening Approaches.
ACS Omega 2, 685–697 (2017)
