MEET US AT AD/PD

We invite you to meet us at the 13th International Conference on Alzheimer’s and Parkinson’s Diseases. The meeting will take place at the Austria Center Vienna – ACV from March 29 to April 2, 2017.
The high-quality scientific program of the AD/PD conferences with a strong focus in mechanisms of disease, prevention and therapy, the interactive and collegial environment that encourages interaction, exchange of ideas and networking among all participants, make the uniqueness of the AD/PD meetings.
Bachem supports its customers in the pursuit of groundbreaking discoveries that further scientific advances, particularly in the field of medicine. We are pleased to present at the conference the latest additions to our broad offer of peptides for Alzheimer’s disease research. Our research products portfolio contains over 240 Alzheimer’s disease-related products available from stock. A comprehensive catalog of biochemicals deliverable ex-stock, an exclusive custom syntheses service for research labs and a full range of services to the pharma and biotech industries complete our service portfolio.
We are excited to meet with our customers, learn their needs for peptides and discuss how Bachem can help to advance their research.
We invite you to visit us at our Booth #52: please contact us to schedule a meeting in advance.
We look forward to meeting you at AD/PD 2017!
AMYLOID PEPTIDES AND ALZHEIMER‘S DISEASE
Alzheimer‘s disease (AD) is the prevalent cause of dementia in elderly people and has become one of the leading causes of death in developed countries. The growing number of AD patients is due to the increased life expectancy: the frequency of AD is about 1% at the age of 60-64 and doubles approximately every five years. AD is an irreversible and progressive neurodegenerative disorder. Symptoms include gradual loss of cognitive functions such as memory, verbal and visuospatial abilities, and changes in personality, behavior, and activities of daily living. AD patients in the final stages completely depend on the care of others.
The characteristic lesions in the brains of AD patients were first described by the German neuropsychiatrist Alois Alzheimer in 1906 during the post-mortem examination of a mentally ill patient. The lesions consisted of dense extracellular deposits, now designated as neuritic or senile plaques, and intracellular dense bundles of fibrils, the neurofibrillary tangles. Currently, diagnosis of AD with adequate testing is approximately 90% accurate. It is based on the exclusion of a variety of diseases causing similar symptoms and a careful neurological and psychiatric examination, as well as neuropsychological testing. Imaging technologies for detecting amyloid plaques and tangles in vivo have become a valuable additional tool. Numerous potential biomarkers as α1-antitrypsin, complement factor H, α2-macroglobulin, apolipoprotein J, and apolipoprotein A-I for diagnosing AD are being evaluated. However, post-mortem histopathological examination of the brain still is the only definite diagnosis of AD.
AD can be either sporadic or inherited. Inherited or familial AD is less common than the sporadic variant comprising 5-10% of all cases. Autosomal dominant mutations in the amyloid β/A4 protein precursor (APP) gene on chromosome 21 and the presenilin-1 or -2 genes on chromosomes 14 and 1, respectively, have been attributed to early-onset AD (before the age of 65). APP is a type-1 integral membrane glycoprotein. More than 10 APP isoforms generated by alternative splicing of the 19 exons have been discovered, APP695, APP751, and APP770 being the predominant ones. Numerous mutations within the APP gene have been detected in families with an inherited risk for early-onset AD. Usually, they are named after the region of first occurrence, e.g. the Swedish double mutation (K670N/M671L), the Dutch mutation (E693Q), or the Austrian T714I mutation (see figure). Mutations in the proximity of enzymatic cleavage sites of APP such as the Swedish mutation and mutations of positions 692-694 (Aβ 21-23), which strongly influence Aβ aggregation, have been studied intensively. The presenilins, another group of proteins involved in the development of AD, are integral membrane proteins with eight transmembrane domains localized in the endoplasmic reticulum and the Golgi apparatus.
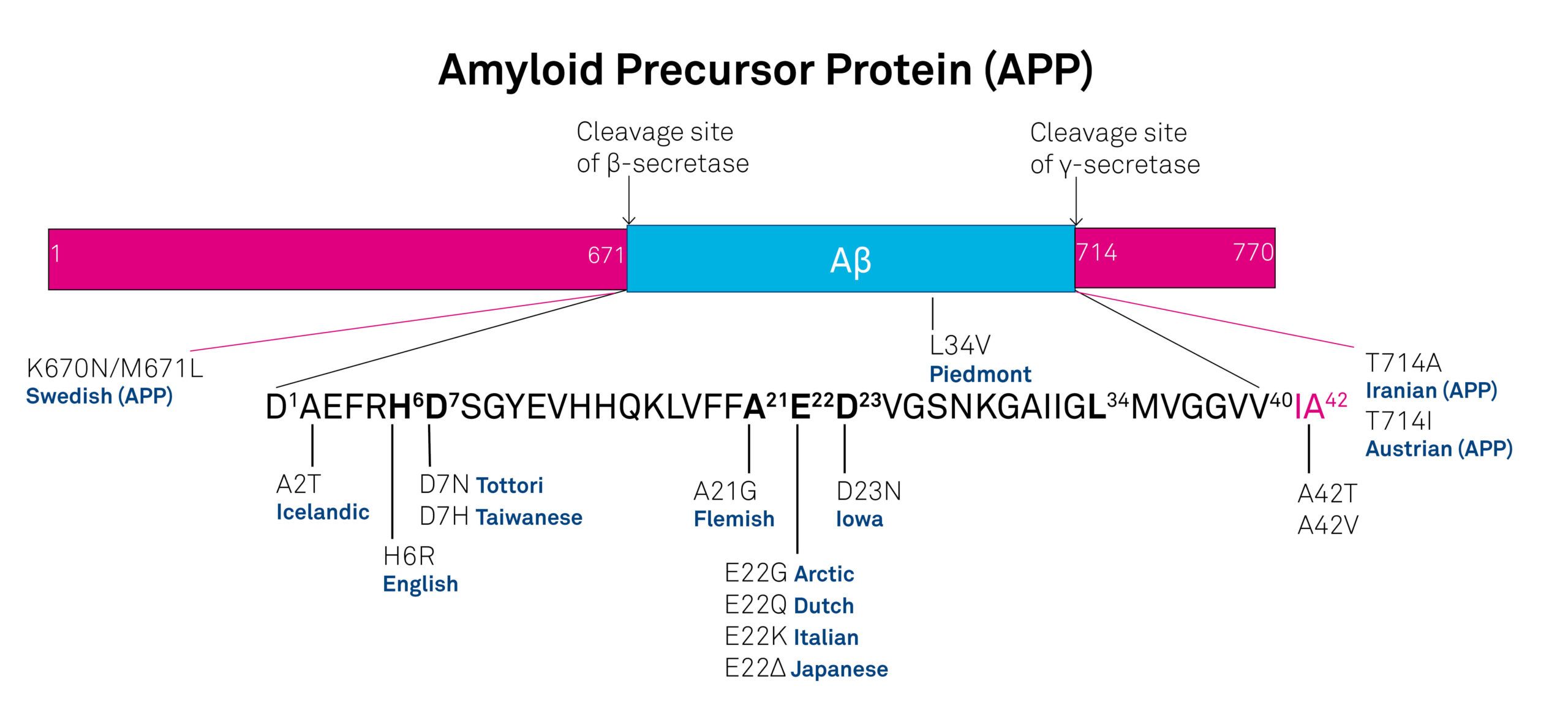
Figure: Mutants in the amyloid-β peptide region and at the cleavage sites of the amyloid precursor protein.
Genetic factors may contribute as well to the late onset of AD. Increased susceptibility is associated with the expression of different apolipoprotein E (ApoE) isoforms due to the polymorphism in the APOE gene on chromosome 19. ApoE in involved in the maintenance of the central nervous system. Carriers of APOEε4 show a higher risk in developing AD than carriers of the alleles APOEε2 and APOEε3. Additionally, polymorphisms of the α2-macroglobulin gene on chromosome 12 and the gene coding low-density lipoprotein receptor-related protein 1 (LRP-1), LRP1-C/T, could increase the susceptibility for late-onset AD.
The etiology of AD is still not completely understood. Initial research focused upon determining the molecular structure of the senile plaques and the neurofibrillary tangles characteristic of AD. The main constituents of the plaques were identified as cleavage products of APP of varying length, the amyloid β-peptides (Aβ peptides). APP can be processed by (at least) three types of proteases, α-secretase, β-secretase (or β-site APP-cleaving enzyme, BACE), and γ-secretase, usually either by α- and γ-secretase (non-amyloidogenic cleavage) or by β- and γ-secretase (amyloidogenic cleavage). Amyloidogenic processing yields the two major constituents of senile plaques, amyloid β-peptide (1-40) (Aβ40) and amyloid β-peptide (1-42) (Aβ42). BACE splits off the extracellular N-terminal protein fragment of APP, soluble APP-β (sAPP-β). The membrane-retained C-terminal part is processed by γ-secretase yielding either Aβ40 or Aβ42. Amyloidogenic processing is a normal process. Both Aβ40 and Aβ42 can be detected in the plasma and cerebrospinal fluid (CSF) of healthy subjects. Whereas similar concentrations of Aβ40 have been measured in the CSF of both healthy controls and AD patients, Aβ42 concentrations in the CSF of AD patients are significantly lower, probably due to deposition as plaques.
The neurofibrillary tangles found inside neurons of Alzheimer’s brains are composed of paired helical filaments whose main components are hyperphosphorylated forms of tau, a microtubule-associated protein involved in promoting microtubule assembly and stabilization. The excessive phosphorylation could be due to the enhanced activity of protein kinases or to the decreased activity of phosphatases.
Accumulation of Aβ42 in the brain is one of the primary events in the development of AD. Most mutations within the APP and the presenilin genes of familial AD manifest as increased cerebral Aβ production. In patients with Down syndrome (trisomy 21), elevated levels of APP and Aβ due to a third copy of the APP gene result in deposition of Aβ at an early age between 20 and 30. Formation of neurofibrillary tangles is considered as a consequence of Aβ deposition with a further impact on the progression of the disease possibly due to disruption of axonal transport mechanisms in neurons.
Several approaches for managing AD have been developed. One of them aims at reducing Aβ40 and Aβ42 by inhibiting the enzymes of the amyloidogenic pathway or by clearance of Aβ by immunization with these peptides. Transition metals as Cu, Fe and Zn play an important role in the pathology of AD. Aggregation and neurotoxicity of Aβ depend on the presence of copper, so Cu-chelating agents showed promising effects in animal models. Another approach is the prevention of the cellular inflammatory response in the cerebral cortex elicited by the progressive accumulation of Aβ. Cholesterol-lowering drugs such as statins and estrogen replacement therapy could reduce the risk of developing AD. Inhibition of glycogen synthase kinase 3 (GSK3) and cyclin-dependent kinase 5 (CDK5), which are involved the phosphorylation of the tau protein, is evaluated as is inhibition of calpain. Increased concentrations of calpain have been detected in AD brains, they promote activation of CDK5. Acetylcholinesterase inhibitors such as tacrine, donepezil, rivastigmine, and galantamine used for treating mild to moderate AD act by reducing the deficits of the neurotransmitter acetylcholine associated with cognitive impairmen. Memantine, an NMDA receptor antagonist, is applied in cases of moderate to severe AD. The β-secretase inhibitors verubecestat (MK-8931) and AZD3293 showed encouraging results in clinical studies. Antibodies as aducanumab and solanezumab, which degrade plaques and lower the level of Aβ in the brain, are under clinical evaluation for mild cases of AD.
Despite many promising therapeutic approaches, AD still remains a major burden for the patients, their relatives, and the society.
Explore our broad offering of amyloid and tau peptides and further technical information
Parkinson’s Disease and Multiple Sclerosis
References
D.R. Thal et al., Capillary cerebral amyloid angiopathy identifies a distinct APOE epsilon4 associated subtype of sporadic Alzheimer’s disease. Acta Neuropathol., 120(2), 169-83 (2010)
W. Danysz and C.G. Parsons, Alzheimer’s disease, beta-amyloid, glutamate, NMDA receptors and memantine-searching for the connections. Br J Pharmacol, 167(2), 324-52 (2012)
P. Anand and B. Singh, A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharm. Res., 36(4), 375-99 (2013)
S.J. Kiddle et al., Candidate blood proteome markers of Alzheimer’s disease onset and progression: a systematic review and replication study. J. Alzheimers Dis., 38(3), 515-31 (2014)
R.M. Lane and T. Darreh-Shori, Understanding the beneficial and detrimental effects of donepezil and rivastigmine to improve their therapeutic value. J. Alzheimers Dis., 44(4), 1039-62 (2015)
S.P. Duggan and J.V. McCarthy, Beyond gamma-secretase activity: The multifunctional nature of presenilins in cell signalling pathways. Cell Signal, 28(1), 1-11 (2016)
D.J. Marciani, A retrospective analysis of the Alzheimer’s disease vaccine progress – The critical need for new development strategies. J Neurochem, 137(5), 687-700 (2016)
F. Panza et al., Tau-Centric Targets and Drugs in Clinical Development for the Treatment of Alzheimer’s Disease. Biomed Res Int, 20163245935 (2016)
H. Potter et al., Role of Trisomy 21 Mosaicism in Sporadic and Familial Alzheimer’s Disease. Curr Alzheimer Res, 13(1), 7-17 (2016)
D.J. Selkoe and J. Hardy, The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med, 8(6), 595-608 (2016)
W.V. Graham et al., Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu Rev Med, 68413-430 (2017
K. Ito et al., Memantine reduces the production of amyloid-beta peptides through modulation of amyloid precursor protein trafficking. Eur J Pharmacol, 79816-25 (2017)
AMYLOID AND TAU PEPTIDES IN CLINICAL DEVELOPMENT
The two major pathological hallmarks of Alzheimer’s disease (AD) are amyloid plaques mainly composed of deposited Amyloid β (Aβ) peptide and the accumulation of neurofibrillary tangles formed from hyperphosphorylated tau protein (1). Accordingly, Aβ peptide has become an important therapeutic target in AD. Immunization against amyloid peptides is one strategy that has been explored; however, the first Alzheimer vaccine to reach clinical trials, AN1792, was unsuccessful. Nevertheless, several new active Aβ peptide vaccines are in development and have since progressed to clinical trials. In addition, tau-based therapies have been the subject of increased interest in recent years and a couple tau peptide containing candidates have reached clinical development as well. An AD vaccine may be on the horizon. There are several Aβ peptide vaccines for the treatment of AD in early clinical development as shown in Table 1. Likewise, tau vaccines that have entered the clinical phases are shown in Table 2.
Table 1: Amyloid Peptides in Clinical Phases of Development (2)
| Product Name | Active Ingredient | Condition Treated | Highest Phase | Companies |
|---|---|---|---|---|
| ACI24 | -- | Alzheimer's Disease(II), Down Syndrome(I) | Phase II | AC Immune SA, Bayer Ag |
| CAD106 | amilomotide | Alzheimer's Disease(II) | Phase II | Kuros Biosciences AG, Amgen Inc, Novartis AG |
| UB311 | -- | Alzheimer's Disease(II) | Phase II | United Biomedical Inc |
| ABvac40 | amyloid beta 40 peptide | Alzheimer's Disease(I) | Phase I | Araclon Biotech SL, Grifols International SA |
| ABVac42 | amyloid beta 42 peptide | Alzheimer's Disease(I) | Phase I | Araclon Biotech SL, Grifols International SA |
| ACC002 | amyloid beta peptide | Alzheimer's Disease(I) | Phase I | Pfizer Inc, Wyeth |
| AD03 AFFIRIS | amyloid beta peptide epitope | Phase I Alzheimer's Disease(I) | Phase I | AFFiRiS AG, GlaxoSmithKline plc |
| LuAF20513 | -- | Alzheimer's Disease(I) | Phase I | H. Lundbeck A/S, Otsuka Pharmaceutical Co Ltd |
Table 2: Tau Peptides in Clinical Phases of Development (2)
| Product Name | Active Ingredient | Condition Treated | Highest Phase | Companies |
|---|---|---|---|---|
| AADvac1 | -- | Alzheimer's Disease(II) | Phase II | Axon Neuroscience SE |
| ACI35 | -- | Alzheimer's Disease(I) | Phase I | AC Immune SA, Janssen Pharmaceuticals Inc |
Phase II Candidate
ACI24 is a liposome-based vaccine being developed by AC Immune. The vaccine contains Aβ peptide (1-15) and is designed to produce beta-sheet conformation-specific antibodies that prevent plaque deposition or enhance clearance. In 2009, AC Immune initiated a Phase I/IIa study to evaluate the safety, tolerability and efficacy of ACI24 in patients with mild to moderate AD. In 2016, AC Immune initiated a Phase Ib trial in patients with Down syndrome (2).
CAD106 (amilomotide) is an immunotherapy being developing by Novartis for the treatment of AD. This therapeutic vaccine consists of the immunodrug carrier Qb coupled to Aβ peptide (1-6). The vaccine is designed to induce antibodies against Aβ protein to inhibit the formation of plaques while avoiding T-cell autoimmune responses (3). In collaboration with Amgen, Novartis initiated a Phase II study called Generation to evaluate the efficacy of CAD106 and CNP520, Amgen’s small molecule inhibitor of beta-site APP-cleaving enzyme-1, in patients at risk of developing clinical symptoms of AD (2).
United Biomedical also has an immunotherapeutic in development for AD known as UB311. The vaccine utilizes United Biomedical’s UBITh® helper T cell technology and a site-specific epitope to target Aβ peptide (4). In 2015, a Phase II study was initiated to evaluate the safety, tolerability, immunogenicity and efficacy of UB311 in patients with mild Alzheimer’s disease (2).
Axon Neuroscience is developing AADvac1, a vaccine containing a peptide fragment, amino acids 294 to 305 of the tau sequence, coupled to KLH. Axon Neuroscience’s vaccine is designed to stimulate patients’ immune systems to attack dysfunctional tau proteins and thereby stop the progression of AD. A Phase I clinical trial with AADvac1 was completed in 2015 and results from the study showed the vaccine to be safe and well tolerated in the assessed parameters. In addition, treatment with the vaccine induced a robust immune response and average cognition of patients remained stable (5)
Phase I Candidate
Araclon Biotech and Grifols International are developing ABvac40, a product containing Aβ peptide (1-40), as a therapeutic vaccine for the treatment of AD. In 2016, Araclon Biotech reported positive results from a Phase I clinical study of ABvac40 (2). ABvac40 demonstrated a good safety and tolerability profile and produced an immune response in more than 87% of patients who received the product in the trial (5).
In addition, Araclon Biotech is developing Aβ peptide (1-42) as a therapeutic vaccine for Alzheimer’s disease. In 2015, the company initiated a Phase I trial with the Abvac42. A Phase II trial is planned for 2017 (2).
Pfizer is developing ACC002 an immunotherapy for the treatment of Alzheimer’s disease. ACC002 contains Amyloid β peptide. The product was in Phase I clinical trials in 2009 but no recent development has been reported (2).
AFFiRis was also developing an AD vaccine, AD03. In 2011, AFFiRiS completed a Phase I study with AD03 and also initiated a Phase Ib follow-up study to evaluate long-term safety and tolerability of AD03 immunization applied during AFFiRiS 005A. The follow-up study was terminated in 2013 due to organizational reasons. No further development has been reported (2). The product is not currently listed in the AFFiRiS company pipeline and may be discontinued (6).
Lu AF20513 is a novel AD epitope vaccine being developed by Lundbeck and Otsuka. This recombinant protein vaccine is composed of two T helper cell epitopes from Tetanus Toxoid (P30 and P2) and three copies of the B cell epitope of Aβ peptide (1-42) (Aβ peptide (1-12)). The vaccine is designed to induce anti-Aβ antibody production in elderly individuals with pre-existing Tetanus Toxoid-reactive memory T helper cells (7). Lundbeck initiated a Phase I study in 2015 of Lu AF20513 to evaluate safety, tolerability and immunogenicity in patients with mild Alzheimer’s disease.
A Phase I candidate targeting tau is AC Immune’s immunotherapy known as ACI-35. ACI-35 is a liposomal vaccine containing a phosphorylated human tau protein fragment (2). In 2015, AC Immune partnered with Janssen Pharmaceuticals to develop ACI-35 and the product is currently in a Phase 1b clinical study in patients with mild to moderate AD (9).
Conclusion
There are several unique immunization approaches currently under development for treating AD. For researchers and organizations investigating this devastating disease, Bachem offers a wide selection of Aβ peptides and tau peptides at shop.www.bachem.com. Furthermore, Bachem offers a comprehensive custom peptide synthesis service and the production of new chemical entities to assist companies with developing peptide-based therapeutics for AD.
References
(1) H. Cynis et al., Immunotherapy targeting pyroglutamate-3 Aβ: prospects and challenges, Mol. Neurodegener. 11(48), (2016)
(2) Medtrack (2017)
(3) M.R. Farlow et al., Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease, Alzheimers Res. Ther. 7(23), (2015)
(4) UB-311, UBI (2017)
(5) Encouraging results of Axon’s tau vaccine advance Alzheimer’s therapy, Axon Neuroscience (2015)
(6) The results of phase I clinical trial of the Araclon Biotech Alzheimer’s vaccine support its continuation, PR Newswire (2016)
(7) Pipeline, AFFiRiS (2017)
(8) H. Davtyan et al., Immunogenicity, efficacy, safety, and mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer’s disease: prelude to a clinical trial, J. Neurosci. 33(11) 4923-434 (2013)
(9) Products, PEP-Therapy (2017) AD treatment and prevention, AC Immune (2015)
MEET BACHEM: SILVIA MENNER
What is your official job title at Bachem?
Inside Sales Representative
How long have you been with Bachem? Where did you work before Bachem?
I have joined Bachem in October ’15. Previously I was at GEA Pharma Systems AG.
Briefly, what do you do at Bachem?
I’m in the Inside Sales department and responsible for several European and overseas clients as far as inquiries, orders or general information for catalogue products are concerned.
What is your academic background/degrees or training?
I studied English and French in Saarbrücken/Germany and Strasbourg/France as translator with a focus on business economics.
What do you like to do outside of work (interests, hobbies)?
I’m in a Line Dance group and read quite a lot, with my family and friends we often go to the cinema or enjoy our spare time together.
What makes a perfect day for you?
I’m happy if all my tasks have been completed and I pass a relaxed evening.
What do you like most about your job?
I’m in contact with many people, my work is made easy because of a system, that provides all relevant information I need.
Have you had any particular expectation when you came to Bachem and have these been fulfilled?
I hoped to work in a team which is fun and where everybody is dedicated to his/ her work. This has become reality and I’m grateful about it.
Thank you very much Silvia.

Peptide highlights
Interesting news about peptides in basic research and pharmaceutical development:
Surprising amyloid structure solved-Chemical & Engineering News
UAB developing new peptide to combat a disorder that causes heart attacks at early age-University of Alabama at Birmingham
New peptide could improve treatment for vision-threatening disease-EurekAlert!
Peptide reverses cardiac fibrosis in a preclinical model of congestive heart failure-EurekAlert!
LITERATURE CITATIONS
Bachem peptides and biochemicals are widely cited in research publications. Congratulations to all our customers with recent publications!
M. Calvo-Rodriguez et al.
Aging and amyloid β oligomers enhance TLR4 expression, LPS-induced Ca2+ responses, and neuron cell death in cultured rat hippocampal neurons.
J. Neuroinflammation, 14(1), 24 (2017)
E. Flores-Martinez and F. Pena-Ortega
Amyloid beta Peptide-Induced Changes in Prefrontal Cortex Activity and Its Response to Hippocampal Input.
Int. J. Pept., 20177386809 (2017)
A. Hategan et al.
HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity.
Nat. Struct. Mol. Biol., (2017)
D.M. Vadukul et al.
Amyloidogenicity and toxicity of the reverse and scrambled variants of amyloid-beta 1-42.
