Solid phase peptide synthesis (SPPS) is a technique for peptide synthesis. It can be defined as a process in which a peptide anchored by its C-terminus to an insoluble polymer is assembled by the successive addition of the protected amino acids constituting its sequence.
Each amino acid addition is referred to as a cycle consisting of:
- a) cleavage of the Nα-protecting group
- b) washing steps
- c) coupling of a protected amino acid
- d) washing steps
As the growing chain is bound to an insoluble support the excess of reagents and soluble by-products can be removed by simple filtration. Washing steps with appropriate solvents ensure the complete removal of cleavage agents after the deprotection step as well as the elimination of excesses of reagents and by-products resulting from the coupling step.
For a general scheme of SPPS see below
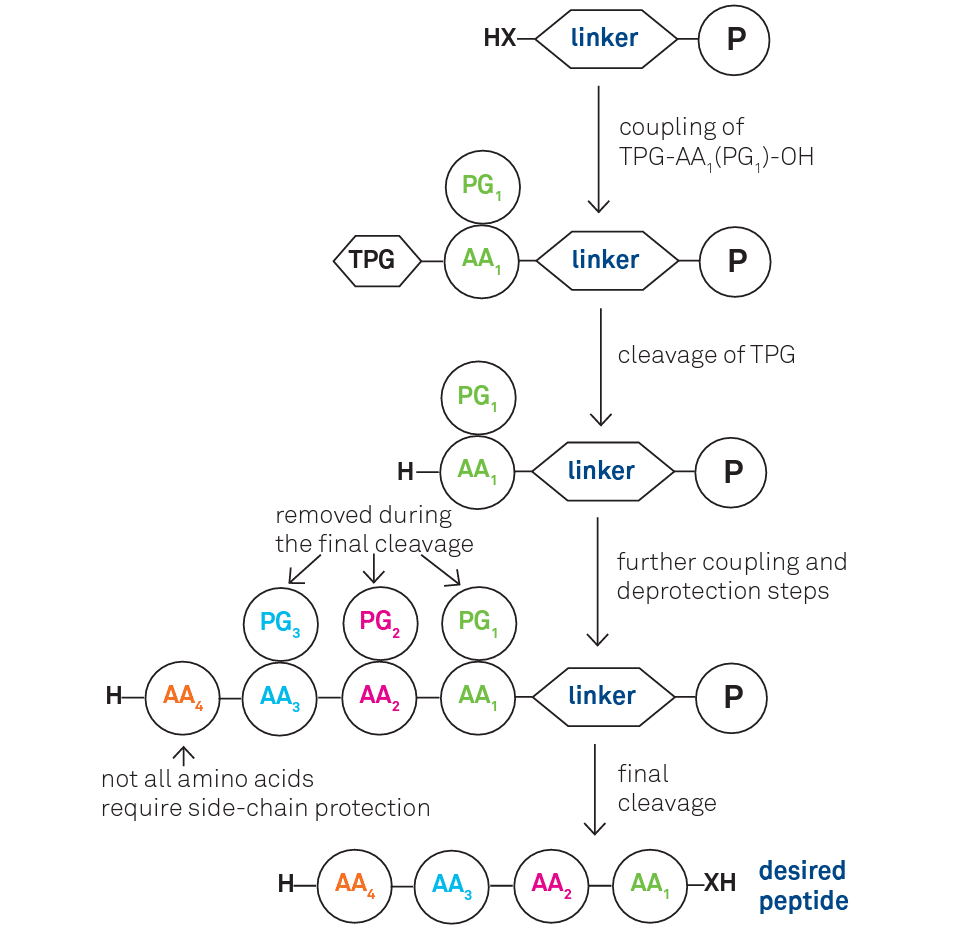
X = O, NH, AA = Amino Acid, PG = Protecting Group, P = Polymer Support, TPG = Temporary Protecting Group
Once the sequence has been completed, the peptide must be cleaved off the resin. Although in general acidolytic cleavage from the resin is the method of choice to release the peptide at the end of the synthesis, a broad range of resins susceptible to be cleaved by nucleophiles such as the «Kaiser oxime resin» and the p-carboxybenzyl alcohol linker or by photolysis has gained popularity.
Quite often, these moieties are not compatible with the conditions of Fmoc SPPS, whereas allyl-based anchors are resistant towards the cleavage conditions of Boc as well as Fmoc protecting groups. The so-called «safety-catch linkers» are perfectly compatible with both Boc and Fmoc chemistries. Only after an activation step they are highly sensitive towards nucleophiles e.g. the sulfonamide linker or 4-hydrazinobenzoic acid.
Boc or Fmoc
The choice of an adequate combination of protecting groups/solid support is the first step on the way to achieve a successful synthesis. For standard SPPS this choice is generally limited to a Boc/benzyl or a Fmoc/tBu based scheme. During the first 15 years of SPPS, the Boc group has been used almost exclusively.
Even if this technique permitted remarkable synthetic achievements the introduction of a new type of protecting group has offered more flexibility for the modification of the peptide chain and/or more specificity in the cleavage of the Nα– versus the side-chain protecting groups.
The combination Fmoc/tBu has met these requirements and broadened the scope of SPPS. Moreover, the development of new resin derivatives has allowed the cleavage of fully protected sequences which can be further coupled in SPPS or in a classical solution process.
In addition, a variety of selectively cleavable protecting groups offers new perspectives for «on-resin» modification (cyclization, formation of disulfide bridges, derivatization of side chains, etc ).
Manual Synthesis
The «classical» reactor for SPPS merely consists of a cylindrical vessel with a fritted disc and a removable lid equipped with a mechanical stirrer. The resin may also be stirred by bubbling nitrogen through, however more elaborate equipment is required. For rapid small scale synthesis a small fritted glass funnel is sufficient. Oxygen and moisture need not be strictly excluded, but the cleavage of the Nα protecting group should be performed under a hood as to avoid exposure to piperidine (Fmoc cleavage) or TFA (Boc cleavage).
The swelling of the resin has to be taken into consideration in the choice of the reactor size. Normally, the volume of the swollen peptide resin will slowly increase during chain elongation. When synthesizing a medium-sized peptide (20–30 AA) using Fmoc SPPS, a 100–150 ml reactor will suffice for ca. 10 g of resin. The swelling will be more important in Boc SPPS mostly during the TFA deprotection step; a 250 ml reactor would be recommended for the above- mentioned synthesis.
At the beginning of each coupling cycle, deblocking or washing step the resin and the solution have to be mixed thoroughly, followed by slow stirring or shaking for the remaining process. All the beads have to be suspended in the liquid for thorough washing, efficient coupling, and complete deblocking. It is important to watch for beads sticking to the wall of the vessel especially during the coupling and rinse them from the wall with a small amount of solvent if necessary. «Sticking beads» may become a problem when stirring too vigorously. Silylation of the glassware improves the surface hydrophobicity and prevents the beads from sticking to the wall of the vessel.
Solvents are filtered off by slight suction, or, more gently, by applying inert gas pressure. In Fmoc/tBu based SPPS the vessel may also be used for the final cleavage or for the cleavage of fully protected peptides from very acid-labile resins.
Continuous Flow synthesis
In this approach the solid support is packed into a column and the reagents and solvents are delivered by a pump. The resins used in this technique must be able to withstand considerable pressure and, at the same time, keep a constant volume while changing solvents. The standard polystyrene-based resin is not suitable for that purpose as the volume of the beads markedly depends on the solvent. This type of peptide synthesizer is best used for Fmoc-based protocols. The Boc protocols generate ionic species during the Boc cleavage, which cause considerable changes in swelling due to electrostatic forces. A synthesizer has been developed in which swelling is monitored, considering that during Fmoc-SPPS, volume changes in a given solvent can only be caused by the growing peptide chain.
Composite material made from a rigid support such as Kieselguhr particles or large pore crosslinked polystyrene in which dimethylacrylamide has been polymerized are used for continuous flow synthesis. Poly(ethylene glycol)-based supports such as TentaGel or PEGA have been introduced for batch as well as continuous flow synthesis.

Fully Automated SPPS
A variety of fully automated synthesizers for batchwise and continuous flow SPPS is commercially available. Fully automated synthesizers employing microwave irradiation for accelerating the synthetic steps were successfully introduced to the market.
Fmoc/tBu SPPS permits automatic monitoring and adequate adjustment of deprotection and coupling times in order to achieve complete conversions. The monitoring relies on strong chromophores which are either released during deprotection or «consumed» (the Fmoc amino acid derivative) and concomitantly released (HOBt or HOAt) during coupling. Monitoring via changes of conductivity allows the monitoring on a real-time basis and end point value can be given to determine the completion of the coupling reaction.

Resins
All resins at Bachem are obtained from beaded polystyrene crosslinked with 1% divinylbenzene (a mixture of the meta and the para isomer). This degree of crosslinking is optimal for SPPS. A higher level of crosslinking would reduce the swelling whereas a decrease would cause a considerable loss of mechanical stability in the swollen state.
The carrier resins for SPPS are obtained from this polymer or from the chloromethylated material. In the second case, the available load is restricted by the degree of chloromethylation. The average bead size is adjusted by the conditions of polymerization. Bachem offers the most popular size distribution 200–400 mesh (average diameter 38–75 μm). A variety of resin derivatives is also available as large beads: 100–200 mesh (average diameter 75–150 μm). With such resins, reaction times may have to be prolonged due to limited diffusion towards the interior of the beads.
The load of the resins is adapted to the needs of routine SPPS: 0.7–1 mEq/g before the loading of the first Fmoc amino acid. Loads may be deliberately reduced, e.g., for side-chain cyclization, for the synthesis of long peptide chains (above 30–40 residues), or for the preparation of sequences presenting intrinsic difficulties. Resins having a particularly high load can be prepared by Bachem on request.
Linkers
Linkers are bifunctional molecules anchoring the growing peptide to the insoluble carrier. Linkers may be coupled to any carrier suitable for SPPS, an important option if alternatives to polystyrene-based resins have to be considered.
The C-terminal Fmoc amino acid may be coupled to the linker yielding the so-called handle which can be purified before loading the polymer. High loads regardless of the bulkiness of the amino acid are obtained by coupling these handles.
What are other techniques to synthesise Peptides
Other techniques used at Bachem are:
LPPS
A classical method of peptide synthesis. In the majority of labs which use it for smaller scale it has been replaced by the solid phase synthesis. In large scale production it has benefits in the manufacturing of peptides for commercial usage.
Molecular Hiving™
Used to produce shorter peptides without hazardous solvents, with more efficient scale-up and enhanced process controls. To learn more on this Innovative synthesis method visit the blog “Innovative approaches to large-scale peptide production“, download the Innovations for Tides Brochüre in our Knowledge Center or rewatch one of the Webinars.
Let’s get in touch!
We’d like to encourage you to reach out to us if you have questions about a project you are currently working on.
Click here >>
Knowledge Center
Visit the Bachem Knowledge Center to download further information about peptides and oligonucleotides
Click here >>
Subscribe to our general newsletter
"*" indicates required fields
