Take your breakthroughs to market with complete confidence – backed by the leading tides partner with 50 years of peptides experience.
Go from inspired drug discovery to global growth, with research-grade material, refined process development and efficient GMP-grade large-scale production.
Perfect your Peptide Product
From simple peptides to the most complex peptidomimetics or synthetic proteins, our pipeline of customer peptide NCE projects includes both analytical services and process development:
Analytical services
- Product characterization
- Development and validation of analytical methods
- Stability studies
- Identification of impurities
Process development, characterization and qualification
- For all phases of clinical trials
- Commercial supply
Reach out to us and discover how you can achieve your goals.
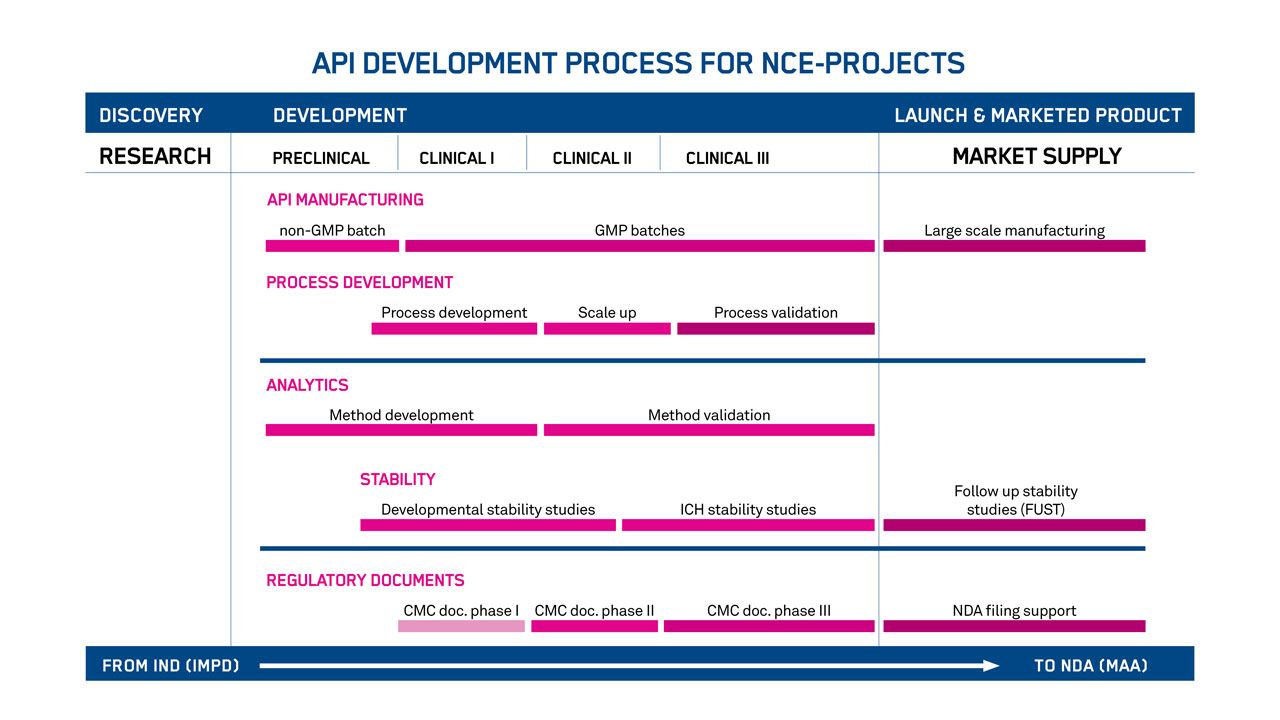
Smoothing the journey to market
Avoid the risks involved with clinical and commercial milestones. We’ll formulate a clear plan for developing the chemistry, manufacturing, and control (CMC) of your peptide and oligonucleotide drug substances.
Years of interactions with regulatory authorities and sponsors around the world have gone into our general plan (below). But we’ll tailor the exact plan to precisely fit your needs – and your product.
Innovating for you
Innovation is part of our DNA and is more important now than ever, as it’s common to see modified or augmented peptide constructs. Peptides with unnatural amino acids, PEGylation, lipidation, glycosylation, imaging agents and structural constraint, are just few examples of what we encounter with today’s peptide-based entities.
We practise innovation by optimizing our supply chain, manufacturing unnatural amino acid derivatives in-house and meticulous characterization at intermediate stages. We also carefully evaluate peptide synthesis methodology, including liquid phase peptide synthesis, solid phase peptide synthesis or hybrid approaches.
Your business and products will also benefit from our toolbox of pioneering commercial manufacturing technology for peptides. From modern synthetic innovations like Molecular Hiving™ to continuous chromatography technology and an eco-conscious overall process, our approach is cutting edge.
Plus, our research committee is constantly evaluating new trends and technologies, and collaborating with academic groups and industry non-profits. So you’ll benefit from the very latest thinking and practices in peptides.
Why work with us?
- Excellence – We offer the highest quality products and services in the industry, with broad knowledge and expertise in peptide development and production.
- Internationally approved – Our facilities are regularly inspected by the FDA, Swissmedic and other prominent regulatory authorities – and we’re fully GMP compliant
- Expertise – Our people are highly skilled and passionate about emerging technologies, but also pride themselves on being reliable and committed to customers.
- Sustainability – We’re convinced that ethical behavior and integrity are essential for long-term business success, so we’re focused on improving our social, economic and environmental sustainability.

What our customers are saying
Peptide News

A conversation with the founders of ChromaCon

The Latest Trends in Peptides and Oligonucleotides

Octreotide acetate – a complex molecule for a complex disease
Next peptide events coming up
RNA Leaders Europe Congress 2024
RNA Leaders Europe Congress 2024
Bachem participates at the RNA Leaders Europe Congress 2024, March 13-14 in Basel
CPhI North America 2024
CPhI North America 2024
Meet Bachem at the CPhI North America at Philadelphia in May 7-9. The event will take place at Pennsylvania Convention Centre.
Webinar: Mitigating supply chain risks in an age of disruption
Webinar: Mitigating supply chain risks in an age of disruption
Learn how Bachem ensures steady operational processes in an uncertain, complex, volatile and complex world.
Day of Life Sciences
Day of Life Sciences
Meet Bachem at the Day of Life Sciences at the School of Life Sciences and Facility Management on June 6th.
Full event calendar
Interested in joining at one of our exhibitions, conferences or webinars?
Peptide knowledge
Our Knowledge Center has a wide variety of tools and materials for peptide chemistry.
Peptide
calculator
Take a look >>
White
papers
Take a look >>
Webinars
Take a look >>
You might also be interested in…
Quality &
Regulatory
Take a look >>
GMP
Production
Take a look >>
Analytical
Capabilities
Take a look >>
Contact us