The peptide NCEs drug market has experienced significant growth in recent years and is expected to continue its upward trend in the coming years.
This is supported by a strong pipeline of peptide drugs in development, with many expected to be approved for use in the coming years. In addition to an increasing pipeline, the manufacturing capacity needed for the peptide API is also growing. Indeed, the advent of oral and inhaled peptide products has driven demand for these active pharmaceutical ingredients (APIs).
Between 2018 and 2020, the average peptide manufacturing batch size increased 2.3 times in response to a wider variety of peptide drug modalities entering the market. Furthermore, with the launch of new GLP-1 analogs that target metabolic diseases like diabetes and obesity, the need for large-scale manufacturing of these APIs to address large patient populations is growing.
Pharmaceutical companies are now under pressure to meet growing needs of generating several hundred kilograms (kg) of peptide active pharmaceutical ingredients (APIs) per year. One significant challenge currently stands in the way of achieving this goal: providing a sustainable, “green” process for large-scale production. Despite the promise of revolutionary new peptide-based therapeutics, producing the necessary quantities of API is a significant challenge, because of chemicals handled.
For example, when producing glucagon-like peptides (GLPs) – often used in treatments for diabetes and obesity – up to 16,000 kg (about 35273.92 lb) of chemicals could be needed per kg of APIs manufactured. These manufacturing processes use excessive amounts of solvents and produce considerable quantities of waste, especially the purification step.
- Reducing environmental footprint with solvent consumption and waste for large-scale purification
- Having a higher throughput and productivity to speed up manufacturing processes
- Increased yield for a target purity
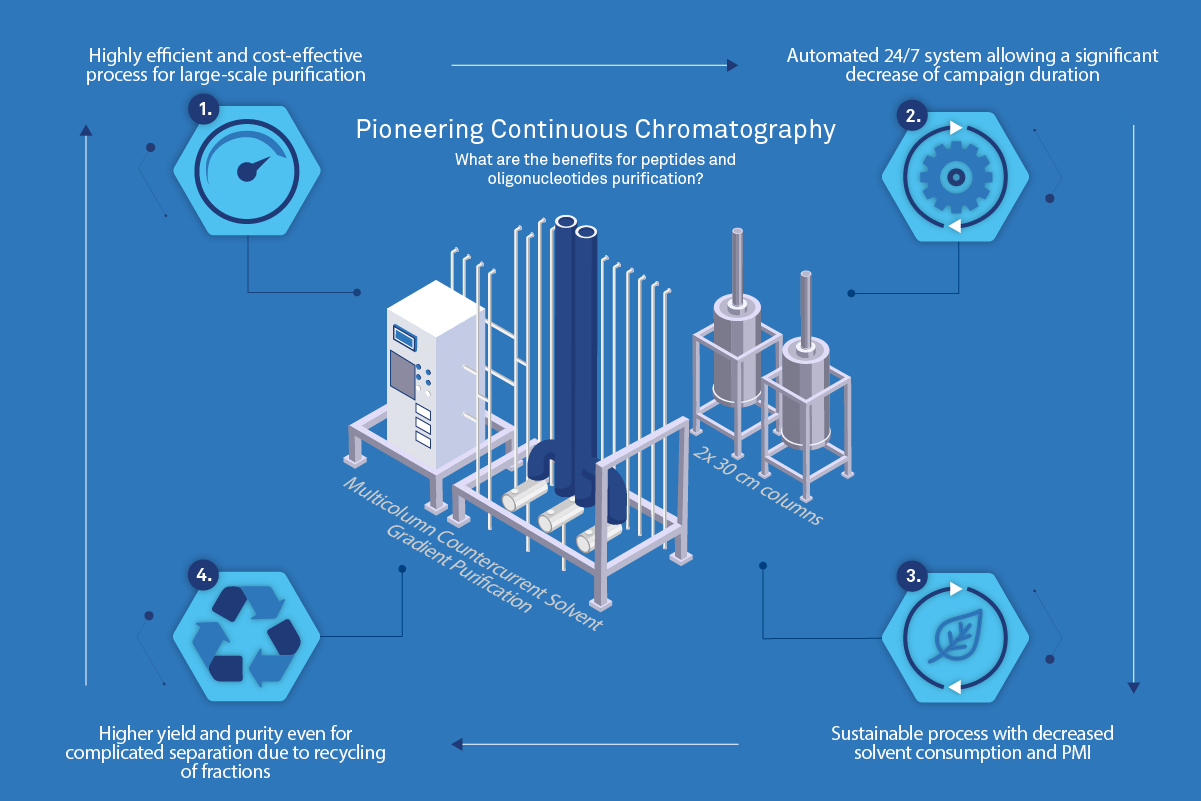
Infographic 1 – Pioneering Continuous Chromatography
This method involves passing the peptide solution through a column filled with a stationary phase, such as silica or beads, and an elution solvent, such as water or buffer. The peptides will bind to the stationary phase, while the impurities will pass through. Chromatography is a highly efficient and selective method of purification, but it can generate a lot of waste and material consumption at large-scale as well as long operation-time.
Bachem’s solution to sustainability and capacity challenges in oligonucleotide and peptide API production was the incorporation of multi-column countercurrent solvent gradient purification (MCSGP) into the downstream process. With this innovative technology in hand, waste, solvents, and operation time are drastically reduced and throughput and productivity are significantly increased.
If you want to learn more on how Bachem is equipped to support the biopharma industry’s growing need for peptide in a more sustainable way, read our case study: click here
Sustainability
Read more about our sustainability efforts.
Click here >>
Let’s get in touch!
We’d like to encourage you to reach out to us if you have questions about a project you are currently working on.
Click here >>
About Bachem
Bachem is a leading, innovation-driven company specializing in the development and manufacture of peptides and oligonucleotides.
With over 50 years of experience and expertise Bachem provides products for research, clinical development and commercial application to pharmaceutical and biotechnology companies worldwide and offers a comprehensive range of services.
Bachem operates internationally with headquarters in Switzerland and locations in Europe, the US and Asia. The company is listed on the SIX Swiss Exchange.
Subscribe to our general newsletter
"*" indicates required fields

